Abstract
Aim
Transanal advancement flap is a recognized technique for complex fistula. Management of the tract is open to discussion. Excision of the tract by the “core out” technique is difficult and could increase the risk of sphincter damage. Curettage is easier but it could increase the risk of recurrence. The aim of the present study was to assess the effect of both techniques on sphincter function and to study the clinical results.
Method
This is a retrospective analysis from a prospective database. One hundred nineteen consecutive patients with high cryptoglandular anal fistula were included. “Core out” technique was performed in 78 patients (group I) and “curettage” in 41 (group II). In both, a full-thickness rectal flap was advanced over the closed internal defect. Anorectal manometry was performed to assess sphincter function. Continence was assessed using the Wexner Scale. Recurrence was defined as the presence of an abscess or fistulization.
Results
Manometric results showed a significant decrease in the maximum resting pressure after surgery in both groups. The maximum squeeze pressure was significantly reduced only in group I (p < 0.001). No significant changes in Wexner score were observed. The overall recurrence rate was 5.88 %, five of group I (6.4 %) and two of group II (4.9 %), without statistical significance (p = 0.74).
Conclusions
The core-out technique causes a significant decrease in squeeze pressures, which reflects damage to the external anal sphincter. This could lead to incontinence in high-risk patients. Curettage is a simple technique that preserves the values of squeeze pressures without increasing recurrence rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endorectal flap advancement procedure is based on the premise that the key to healing is the closure of the high pressure side of the fistula. This technique achieves a secure closure of the internal opening and theoretically preserves the normal anatomy and function of the anal canal. However, the fact remains that the results in terms of success rates and incontinence are heterogeneous (1).
Several surgical procedures have been developed to minimize recurrences and fecal incontinence, including the insertion of a collagen bovine sponge impregnated with gentamicin sulfate beneath an endoanal flap (2) or the fibrin glue obliteration of the fistula tract combined with flap repair (3). Many other changes in surgical technique have also been studied, such as the use of full- or partial-thickness rectal flaps, curved incisions and rhomboid flaps, the closure or non-closure of the internal opening, and the best shape and size of the flap (4–10).
However, the impact that the different ways to treat the fistula tract might have on the results of transanal flaps has not yet been studied. The “core out” technique to treat the fistula tract has been widely used. The coring procedure is rather tedious; it can be very difficult in some tracts and could increase the risk of sphincter damage. Debridement and curettage of the tract is an easier procedure and produces a satisfactory outcome as other authors have shown (10), but it could increase the risk for inadequate drainage and subsequent non-healing (9).
The aim of the present study was to assess the effect of both techniques on sphincter function using anal manometry and to study the clinical results, focusing on recurrence rate and continence.
Patients and methods
We retrospectively analysed a prospectively entered database. From January 1995 to June 2013, 139 consecutive patients were treated using the endorectal advancement flap for complex anal fistula in our institution. Fistulas were registered following Parks’s classification and can be defined as complex fistulas as those with tracts cross more than 50 % of the external sphincter, presence of high secondary tracts or chronic abscess cavities, fistulas associated with Crohn’s disease, and preexisting high risk factors of incontinence. If an associated abscess was detected, a drainage was performed prior to the definitive treatment. Clinical exams and endoanal ultrasounds with B&K Medical completed the preoperative study.
Patients with history of Crohn’s disease, with fecal incontinence prior to surgery and mucosal advancement flaps were excluded in this retrospective analysis. Therefore, all patients included in the study had complex cryptoglandular fistulas with complete preoperative continence and full-thickness flap.
The present series comprised 119 patients, 80 men and 39 women. Their median age at the time of surgery was 50 (range 22–79) years. Eleven patients (9.2 %) had been previously operated one or more times before referral to our hospital or the establishment of Section of Coloproctology.
This medical analysis was approved by a research ethics committee.
Surgical procedure
Standard mechanical bowel preparation alongside antibiotic and antithromboembolic prophylaxis was used. The surgery was performed using regional or general anesthesia, and the positioning of the patient depended on the location of the internal opening: lithotomy in posterior fistulas and jackknife in anterior fistulas. All operations was executed by or under the supervision of the same accredited colorectal surgeon.
Patients of Group I (“core-out” technique)
From 1995 to 2009, “core-out” of fistula tract was the election technique. The primary tract was dissected from the external to the internal opening. After assessing the extent of the fistula, an incision around the skin was made and the incision was developed sufficiently to expose the adjacent borders of the sphincter muscles. After identifying the primary internal opening, a transverse elliptical incision was made around it, covering the full thickness and deepening outwards in cone shape. The main fistulous tract was cored out through the external sphincter muscle. The crypt-bearing tissue around the internal opening of the fistula was excised along with the main tract. Thus, the complete extirpation of the fistulous tract was achieved. (Fig. 1a, b)
Patients of group II(curettage technique)
Since 2010 only curettage has been executed. The tract of the fistula was treated by drainage rather than excision. Skin around the external opening was excised and the granulation tissue lining the tract was curetted with a sharp spoon, taking heed of not damaging the external sphincter. If cavity was associated, this is externally drained using a Pezzer catheter. Subsequently, the internal opening of the fistula was excised along with the cryptoglandular infective focus through the internal sphincter up to the intersphincteric space. The fistula tract in the external anal sphincter was only curettaged.
The other steps of the procedure have been described previously (11) and are the same for both groups. A full thickness of rectal wall was dissected proximally using cutting diathermy to minimize bleeding and advanced over the closed internal defect. Preservation of the integrity of the internal sphincter distal to the internal opening must be of great importance in our experience. Patients were treated with liquid diet during 4 days, followed by normal diet and bulk laxatives.
Follow-up
The data for patients recruited into the study were prospectively collected into database. Demographic characteristics (age, sex, smoking, ASA), previous anorectal operation, type and origin of the fistula (anatomy, horseshoe extensión, supralevator cavity), and surgical outcome (postoperative stay, complications, recurrence, and incontinence) were recorded.
Clinical results were obtained from direct interview and exploration at a minimum follow-up of 12 months. Healing of the fistula was defined as complete wound healing and closure of all external openings in combination with absence of symptoms. Recurrence was defined as the presence of an abscess arising in the area or evidence of fistulization.
All patients were questioned about incontinence symptoms according Wexner Continence Grading Scale (WCGS) (12) before and 3 months after operation. The score can vary from 0: perfect continence to 20: complete incontinence. Continence was considered unaffected when there was no change in continence score and deteriorated when the score decreased after surgery. An increase of one to three points was considered as minor, and of four points or more as a major decrease in continence.
The functional outcome of fistula surgery has been also quantified by anal manometry, preoperatively and 3 months after surgery. The anorectal manometry was conducted by an independent researcher of Digestive Motility Unit of Clinic Hospital of Valencia, and we completed the full study in 93 patients (78.1 %). It was performed using a low compliance water perfusion system (Arndorfer Medical specialties, Greendale, WI, USA) with a four-lumen catheter (Synectics Medical, external diameter, 4 mm; Synectics AB, Stockholm, Sweden), having radially arranged ports in cross section. With the patient in the left lateral position and the hips flexed to 90°, the catheter (lubricated lightly with water-soluble gel) was inserted into the rectum, so that the manometric holes were situated 6 cm from the anal verge. After a 60-s delay, the catheter was withdrawn from the rectum in 0.5-cm steps, remaining for at least 1 min at each station to ensure that the pressure there had reached a plateau, then asking the patient to squeeze maximally. Maximum anal resting pressure (MRP) and maximum squeeze pressure (MSP), obtained as the maximal voluntary anal contraction related to basal rectal pressure. Reference values of our laboratory (healthy volunteers matched by age and sex) were used as normal values.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 for Mac (SPSS, Chicago, IL, USA). Variables are presented as percentage or as mean ± SD and range for all numerical variables. Categorical variables were analyzed using the chi-square Pearson. Continuous measures were analyzed using the Mann-Whitney U test for independent variables and the Wilcoxon test for associated variables. P < 0.05 was considered statistically significant. The cumulative probability of recurrence was analyzed by the Kaplan-Meier method and compared using the log-rank test.
Results
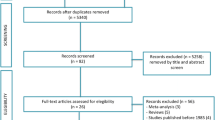
A total of 139 patients underwent endorectal advancement flap repair for anal fistula at this center. Patients with history of Crohn’s disease (n = 4), fecal incontinence prior to surgery (n = 10), and mucosal advancement flaps (n = 6) were excluded from the analysis. Finally, 119 patients with complex cryptoglandular anal fistulas were qualified for inclusion into this study (Fig. 2). Seventy-eight patients were treated with “core out” (group I) and 41 with curettage (group II).
Demographic and clinical characteristics are shown in Table 1. No statistically significant differences between the two groups were found. Fistula type, previous drainage, previous fistula surgery, horseshoe extension, or supralevator cavity were evenly distributed (minimum p = 0.12) (Table 1).
Recurrence
All patients were followed for a minimum of 1 year, and the overall median follow-up period was 23 months. Fistula recurrence occurred in seven patients: five (6.4 %) of group I and two (4.9 %) of group II, giving an overall recurrence rate of 5.9 %, without statistical differences between the two groups (p = 0.74). All recurrences were detected during the first 6 months after operation. The cumulative incidence of recurrent fistula is shown in Fig. 3, with no differences between the 2 groups (log-rank test p = 0.716). Five patients in whom the procedure failed underwent new surgery using the same technique and healed. Two patients recurred with a low intersphincteric fistula, which could be handled with fistulotomy.
Manometric and clinical results
Manometric results are shown in Fig. 4. Resting pressure (MRP) decreased significantly in both groups after surgery. In group I, there was a significant decrease from 87.3 ± 27.4 to 47 ± 20.1 mmHg (p < 0.001) and in group II from 92.8 ± 34.2 to 44.3 ± 20.6 after operation (p < 0.001).
Maximum squeeze pressure (MSP) decreased significantly in group I from 240.3 ± 99 to 189.9 ± 78.9 mmHg after surgery (p < 0.001), but in group II, it did not differ significantly between preoperative and postoperative values (217.3 ± 74.8 to 203.8 ± 77.6 mmHg) (p = 0.1).
Wexner questionnaire data are summarized in Fig. 5. All patients had a Wexner score of 0 at preoperative assessment. At postoperative assessment, a Wexner score of 0 was identified in 76.5 % of all scored patients, 59 patients of group I (75.6 %) and 32 of group II (78 %) (p = 0.3). The proportion of patients with a postoperative minor increase of the Wexner score was 19.2 % in group I and 22.0 % in group II, while 4 (5.1 %) patients of group I and none of group II had a major increase of the Wexner score postoperatively.
In particular, in the four patients with a major increase of the Wexner score, the preoperative MSP was significantly lower than the other patients in group I (139 ± 29.3 vs 248.2 ± 98.3, p = 0.02), maybe defining a group of patients at high-risk for incontinence.
Discussion
The outcome of patients with complex fistulas and the actual value of the procedure are determined by two factors: anal continence and recurrence. The technique of rectal advancement flap has the practical advantages of curing and preserving the normal anatomy and function of the anal canal. Good results using this technique have been reported in the literature, but there is a wide range of success rates and heterogeneous results, both in relation to continence or recurrence rates (1, 10, 13–18).
The wide variability of results between different institutions and surgeons is a fact and suggests that technical factors might play a significant role in the outcome of an advancement flap. Several RCT (randomized controlled trials) have failed to show improved outcomes with surgical variations, and there are only a few recognized factors that might increase the risk of failure, such as smoking or concomitant obliteration of the tract with injection of fibrin glue or plugs (2, 3, 19). Some authors have suggested that a full-thickness flap is better than just a mucosal flap, and reported an improvement of recurrence rates without higher incontinence rates when a full mobilization of the rectal wall is performed (4). Likewise, a prospective randomized study (5) concluded that partial thickness is better than mucosal advancement flaps. The modifications of the flap are not the object of this study, but like Lewis (10), we believe that full-thickness flaps of the rectal wall increases the strength and vascularity of the suture sites and provides a thicker cover for the deep end of the fistulous tract. We always design a flap about half a circumference of the anorectal canal, and it is mobilized to cover without tension the closed end of the track. It also seems important to be careful to keep the distal internal sphincter intact, therefore preventing a keyhole deformity of the anal margin that can lead to mucus or stool leakage.
Regarding the fistulous tract, it is common for many surgeons to manage it by “coring out” the tract (8, 9, 16, 20, 21), from the external to the internal opening. The coring procedure is time-consuming, and it would be very difficult in horseshoe tracts. Furthermore, when the tract is cored out through the external sphincter muscle, it may be damaged. Debridement and curettage of the tract is a much easier procedure and some authors have shown satisfactory outcomes (10, 14, 18), but it could increase the risk of inadequate drainage and recurrence (9). However, the comparison of consequences and results of “core-out” or curettage of the fistulous tract have never been analyzed.
Manometry has been performed in few studies on endorectal advancement flaps (6, 9, 22–24). Our results revealed a significant decrease in resting pressure after the advancement flap in both groups. Resting anal pressure mainly reflects the function of the internal anal sphincter, and its decrease indicates involvement of the internal sphincter in the full-thickness flap. Some of the other manometric studies show similar results (6, 9), and this reduction was only present when the flap included the anorectal muscle layer (25). Maximum squeeze pressure is a measure of the external anal sphincter function, and some investigators have found it to decrease after advancement flaps (6, 24). The anal dilation during surgery has been postulated as a possible cause (26). In our experience, MSP showed a significant decrease only in the “core-out” group, whereas no changes were found in the curettage group, which could be explained by sphincter damage when the tract is tunneled from the sphincters.
Avoidance of fecal incontinence is one of the objectives of the different surgical techniques, and the endorectal advancement flap has been associated with a wide inter-study variability between 0 and 35 %, (4, 6, 8, 10, 13, 15, 20). Particularly noteworthy is the frequent omission of specific information in many studies, and as Soltani noted in a review of the literature, systematic evaluation and questioning of patients after transanal advancement flaps might reveal a much higher incidence of fecal incontinence (1). In our study, some symptoms of incontinence were found in 23.5 % of all patients, similar to other reports (4, 17). We report minor symptoms with a slight decrease in continence in 20 %; major disturbances (WCGS score >4) were found only in the “core out” group. Furthermore, we found that patients with major decrease in continence had significantly lower squeeze pressure before surgery than the other patients, despite scoring 0 in the Wexner score. It is possible that the “core-out” was the precipitating factor for incontinence in high-risk patients.
The reported success rates within an average follow-up of 28.9 months show a wide inter-study variability from 36 to 98.5 % (1) with large differences in recurrence rates from 2 to 43 %. We have obtained a mean recurrence rate of 5.8 %. The curettage group did not show worse recurrence rates, and there was no difference between the two treatment groups. Concerning the time point of recurrence, it has been reported that it normally occurs within the first year after surgery (4, 27). In this study, all recurrences were detected during the first 6 months after surgery. Therefore, we also believe that extending the length of follow-up beyond 1 year is not necessary.
To the knowledge of the authors, this is the first study that compares core-out and curettage of the fistulous tract in rectal advancement flap for anal fistula. Although the study is retrospective, data were collected prospectively and the two groups resulted to have similar preoperative characteristics. Moreover, the fact that the same surgeon performed all the procedures, guarantees the uniformity of the surgical technique. Finally, the anal pressure measured by manometry, associated with the evaluation of the continence with a widely validated score system, allowed to assess the functional results of the two surgical techniques.
Conclusions
From the analysis of the data, it can be concluded that the core-out technique causes a significant decrease in squeeze pressures that reflects damage to the external anal sphincter. This can lead to incontinence in high-risk patients. Curettage is a simple technique that does not change the values of MSP, and presents the same recurrence rates.
References
Soltani A, Kaiser AM (2010) Endorectal advancement flap for cryptoglandular or Crohn’s fistula in ano. Dis Colon Rectum 53:486–495
Gustafsson UM, Graf W (2006) Randomized clinical trial of local gentamicin-collagen treatment in advancement flap repair for anal fistula. Br J Surg 93:1202–1207
Ellis CN, Clark S (2006) Fibrin glue as an adjunct to flap repair of anal fistulas: a randomized, controlled study. Dis Colon Rectum 49:1736–1740
Dubsky PC, Stift A, Friedl J, Teleky B, Herbst F (2008) Endorectal advancement flaps in the treatment of high anal fistula of cryptoglandular origin: full-thickness vs. mucosal-rectum flaps. Dis Colon Rectum 51:852–857
Khafagy W, Omar W, El Nakeeb A, Fouda E, Yousef M, Farid M (2010) Treatment of anal fistulas by partial rectal wall advancement flap or mucosal advancement flap: a prospective randomized study. Int J Surg 8:321–325
Koehler A, Risse-Schaaf A, Athanasiadis S (2004) Treatment for horseshoe fistulas-in-ano with primary closure of the internal fistula opening: a clinical and manometric study. Dis Colon Rectum 47:1874–1882
Detry R, Kartheuser A, Remacle G (1994) Traitement des fistules anales profondes par avancement d’un volet de paroi rectale. Ann Chir 48:178–182
Schouten WR, Zimmerman DD, Briel JW (1999) Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum 42:1419–1422
Gustafsson UM, Graf W (2002) Excision of anal fistula with closure of the internal opening: functional and manometric results. Dis Colon Rectum 45:1672–1678
Lewis P, Bartolo DC (1990) Treatment of trans-sphincteric fistulae by full thickness anorectal advancement flaps. Br J Surg 77:1187–1189
Uribe N, Millan M, Minguez M, Ballester C, Asencio F, Sanchiz V, Esclapez P, Ruiz J (2007) Clinical and manometric results of endorectal advancement flaps for complex anal fistula. Int J Colorectal Dis 22:259–264
Jorge JMN, Wexner SD (1992) Etiology and management of fecal incontinence. Dis Colon Rectum 35:482–487
Aguilar PS, Plasencia G, Hardy TG Jr, Hartmann RF, Stewart WR (1985) Mucosal advancement in the treatment of anal fistula. Dis Colon Rectum 28:496–498
Kodner IJ, Mazor A, Shemesh EI, Fry RD, Fleshman JW, Birn- baum EH (1993) Endorectal advancement flap repair of rectovaginal and other complicated anorectal fistulas. Surgery 114:682–689
Ortiz H, Marzo J (2000) Endorectal flap advancement repair and fistulectomy for high trans-sphincteric and suprasphincteric fistulas. Br J Surg 87:1680–1683
Miller GV, Finan PJ (1998) Flap advancement and core fistulectomy for complex rectal fistula. Br J Surg 85:108–110
Ozuner G, Hull TL, Cartmill J, Fazio VW (1996) Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum 39:10–14
Sonoda T, Hull T, Piedmonte MR, Fazio VW (2002) Outcomes of primary repair of anorectal and rectovaginal fistulas using the en- dorectal advancement flap. Dis Colon Rectum 45:1622–1628
Mitalas LE, Van Onkelen RS, Gosselink MP, Zimmerman DDE, Schouten WR (2010) The anal fistula plug as an adjunt to transanal advancement flap repair. Dis Colon Rectum 53:1713
Wedell J, Meier zu Eissen P, Banzhaf G, Kleine L (1987) Sliding flap advancement for the treatment of high level fistulae. Br J Surg 74:390–391
Oh C (1983) Management of high recurrent anal fistula. Surgery 93:330
Lewis WG, Finan PJ, Holdsworth PJ, Sagar PM, Stephenson BM (1995) Clinical results and manometric studies after rectal flap advancement for infra-levator trans-sphincteric fistula-in-ano. Int J Colorectal Dis 10:189–192
Kreis ME, Jehle EC, Ohlemann M, Becker HD, Starlinger MJ (1998) Functional results after transanal rectal advancement flap repair of trans-sphincteric fistula. Br J Surg 85:240–242
Perez F, Arroyo A, Serrano P, Sanchez A, Candela F, Perez MT, Calpena R (2006) Randomized clinical and manometric study of advancement flap versus fistulotomy with sphincter reconstruction in the management of complex fistula-in-ano. Am J Surg 192:34–40
Roig JV, García-Armengol J, Jordán JC, Moro D, García-Granero E, Alós R (2010) Fistulectomy and sphincter reconstruction for complex cryptoglandular fistulas. Color Dis 12:145–152
Zimmerman DD, Gosselink MP, Hop WC, Darby M, Briel JW, Schouten WR (2003) Impact of two different types of anal retrackor on fecal incontinence after fistula repair: a prospective, randomized, clinical trial. Dis Colon Rectum 46:1674–1679
Ortiz H, Marzo M, de Miguel M, Ciga MA, Oteiza F, Armendariz P (2008) Length of follow-up after fistulotomy and fistulectomy associated with endorectal advancement flap repair for fistula in ano. Br J Surg 95:484–487
Acknowledgments
The authors would like to thank Matteo Frasson for his suggestions.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uribe, N., Balciscueta, Z., Mínguez, M. et al. “Core out” or “curettage” in rectal advancement flap for cryptoglandular anal fistula. Int J Colorectal Dis 30, 613–619 (2015). https://doi.org/10.1007/s00384-015-2133-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2133-x