Abstract
Background
The aim of our study is to evaluate the outcome of patients affected by brain metastases from colorectal cancer and to correlate the outcome with prognostic factors.
Methods
Patients were retrospectively evaluated. Survival distributions were estimated by using the Kaplan–Meier method. The log-rank test was used to assess the impact on survival of individual factors.
Results
Among 41 patients (25M and 16F; median age 58), 58.5 % had rectal cancer and 39 % synchronous metastatic disease; 95 % had extracranial metastases, most common site was lung (87.8 %). Seven patients had synchronous brain metastases. Median overall survival after diagnosis of brain metastases was 5 months [95 % confidence interval 3–12 months]. Median survival from brain metastases diagnosis was 4.2 months in patients treated with radiotherapy (29.3 %), 11.9 months in those with radio- and chemotherapy (21.9 %) and 21.4 months in those with surgery with/without radiotherapy or chemotherapy (29.3 %) (P < 0.0001). On multivariate analysis, no independent prognostic factors were found for disease-free interval from diagnosis to brain metastases and overall survival; amount of chemotherapy before brain metastases have no statistically significant relation to brain-metastases-free-interval even if patients who received more than one line of chemotherapy have a longer median brain-metastases-free-interval than those who received less than one. KRAS was found mutated in 17/28 patients without statistically significant correlation to outcome due to the small sample size.
Conclusions
Prognosis of brain-metastases-patients is poor. An interesting tool is to evaluate the correlation of KRAS status and brain metastases with aim to tailor treatment and follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among all intracranial tumors, metastatic brain involvement is reported in 20–40 % of cases and is more frequently associated with lung (50–60 %) and breast cancer (12–15 %). The incidence of brain metastases (BM) associated to colorectal cancer (CRC) is occasionally reported in literature and ranges between 1 and 4 %, even if an increasing signaling was recently observed, due to prolonged survival of patients treated with more effective therapeutic strategies [1].
Therefore, this event is often delayed and occurs in long-term survivors but more rarely an early event of a systemic spread. Sporadically, brain is reported as the only documented metastatic site.
Metastatic pattern within the brain is variable: cerebral locations are more frequent (80–85 %) than cerebellar ones and are solitary in 40–60 % of cases. Due to the infrequence of this event and related lack of well-defined follow-up assessment, patients are often symptomatic at diagnosis. Their outcome is worst despite aggressive approaches such as surgery and/or radiotherapy, and their median survival ranges from 3 to 13 months [2–4]. Among several variables that could influence the prognosis of all patients with BM due to solid tumors, most validated are performance status, extent of extracranial disease, and age [5]. More recently, also, “previous amount of chemotherapy before brain metastasis” was added as an independent prognostic factor for survival [6].
Due to the infrequence of BM associated to CRC, therapeutic plan is not well established and is based on a case-by-case approach; few case reports and retrospective collections of clinical data were reported in literature with the aim to assess natural history, outcome, and possible prognostic factors.
Our intent is to investigate the outcome of patients with BM, to determine the benefit of local and systemic treatments, and to evaluate clinical and biological survival-related parameters.
Patients and methods
Patient population
We have evaluated 41 patients affected by BM from CRC consecutively referred in our institute during the last 10 years.
Computed tomography (CT) or magnetic resonance imaging (MRI) studies confirmed the presence of BM in all cases.
The relevant patients’ records were later reviewed with respect to the clinical variables whose main ones were age, gender, site, stage, and grading of primary tumor and interval from the primary tumor diagnosis and BM and KRAS mutational status. The following variables also were reviewed: the presence and location of other systemic metastases, age at BM diagnosis (≤ or >65 years), and neurologic symptoms at the time of BM diagnosis. By using the CT/MRI findings, the numbers of BM were noted along with the location (cerebral and/or cerebellar).
Treatment
Treatment modalities for BM included surgical resection, whole brain radiotherapy (WBRT), stereotactic radiotherapy (SRT), and systemic chemotherapy plus or not biological agents. The treatment approach was chosen in a multidisciplinary assessment based on number, site and distribution of cerebral lesions, presence of neurological symptoms, extension of extracranial metastases, and previous antitumor therapies. Steroids and other supportive care were given as clinically indicated.
Statistical analysis
Sample baseline characteristics have been described by using mean, standard deviation, minimum and maximum for continuous variables, and frequency tables and percentages for categorical variables.
The survival from CRC diagnosis (OS) and the survival from the time of BM involvement were calculated from the date of diagnosis to death or last known follow-up. BM-free survival from primary tumor was calculated as time interval from diagnosis of primary tumor to metastasis occurrence or last known follow-up. Survival distributions were estimated by using the Kaplan–Meier method. The log-rank test was used to assess the impact on survival of individual factors. The Cox proportional hazards multivariable model included only the variables associated to the outcome at a significance level of P = 0.10. All statistical tests were two-tailed. Statistical analyses have been performed using SAS statistical software (version 9.2 for Windows).
Results
The main patients’ and BM characteristics are described in Tables 1 and 2, respectively. The primary location was the rectum in 24 (58.5 %) while colon was involved in 17 (41.5 %) patients. The grading of primary cancer was G1–G2 and G3 in 20 (48.8 %) and in seven (17.1 %) patients, respectively. Stage at diagnosis was II in 8 (19.5 %), III in 16 (39 %), and IV in 16 (39 %) patients, respectively. Twenty-two patients, among 24 without metastases at diagnosis (91.7 %), received adjuvant chemotherapy after surgery and, among them, eight were treated with oxaliplatin containing regimen. Ten patients received one and eight more than one chemotherapy lines for advanced disease while five patients did not receive any chemotherapy before BM diagnosis. In 13 cases, biological drugs were delivered. Two patients (5 %) had BM as the only apparent location, while 88 % had lung metastases at time of BM diagnosis. In seven cases, the BM was synchronous in 34 metachronous. In 22 patients, there was a single lesion, and in 17, there were multiple; in 12 cases, the cerebellum was involved.
Among 12 patients who underwent surgery as primary treatment for BM, R0 resection of BM was performed in seven cases, while R1/R2 in five cases. Time to BM metastases ranges was similar between 0 and 69 months and between 0 and 70 months in the R0 resection and R1/R2 resection group, respectively. OS ranges were 20–86 and 21–84 months in the R0 resection and R1/R2 resection group, respectively.
The majority of patients (95 %) treated with surgery for BM had diffuse metastatic disease, not suitable to local treatment, at the time of BM appearance. Some of these patients have previously received resections of lung or liver metastases, but at the time of BM metastasization, systemic progression also occurred. Two patients had “only brain” localizations, one with previously resected lung metastases and another one with brain appeared as first metastatic site and subsequent liver spread.
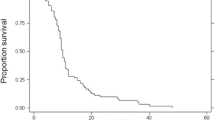
KRAS mutation was evaluated on codons 12 and 13 in 28 patients (either in primary tumor or metastases); among them, 17 (61 %) were found mutated. No statistically significant correlation was found between KRAS status and BM event due to small sample size (data not shown). The median free interval between diagnosis of the primary tumor and metastasization to the brain was 36 months (range 0–116; 95 % CI 27–45). Regarding treatment, 12 (29.3 %) patients received surgery (11 plus radiotherapy and/or chemotherapy), 29 (70.7 %) radiotherapy, among which, 9 stereotactic, 14 whole brain radiotherapy and 5 both of them, 17 (51.2 %) chemotherapy, and 6 (14.6 %) supportive care alone (Table 3). Median OS was 48 months (range 5–118, 95 % CI 42–63) and median survival from diagnosis of BM was 5 months (range 0–51, 95 % CI 3–12). (Figs. 1 and 2). Dukes’ stage and type of BM (synchronous versus metachronous) is associated to better outcome at univariate analysis while no impact on overall survival was found to be related to gender, age, site of primary tumor, grading, timing, and type of BM (Table 5). Again, gender, age, site of primary tumor, and grading have no impact on BM free interval. Dukes’ stage at first diagnosis of colorectal cancer is the only factor statistically significant related to BM free interval (Table 4).
Regarding chemotherapy, adjuvant therapy, and amount of chemotherapy before BM (<1 versus ≥2) have no statistically significant relation to BM free interval even if patients who received more than one line of chemotherapy have a longer median BM free interval than those who received less than one (Table 4). Presence of neurological symptoms, age (> versus ≤65 years), extent of extracranial disease at BM onset are also evaluated in relation to patients’ outcome; only the presence of symptomatic BM is related to worse survival in our study. At the time of evaluation (median follow-up 48 months, range 5–118), 17 patients (51 %) were alive (Fig 1).
Discussion
Metastasization to central nervous system is a rare event in CRC; therefore, there are no prospective controlled trials that have evaluated optimal treatment and outcome. At the same time, randomized studies generally do not include patients with BM, and so, we have no data about the efficacy of chemotherapy and biological agents in this setting of patients.
We have described the outcome of 41 patients affected by BM from CRC referred consecutively to our Institute, with the aim to investigate on the presence of prognostic factors.
Median age of our patients was 58 (range 23–75) years, and the most common site of primary tumor location was the rectum (58.5 %); almost all the patients had an advanced disease at BM diagnosis, among which, 87.8 % had lung involvement, as often reported in other tumors (breast, lung, and renal ones) and in other retrospective studies in CRC series [1, 2].
We consider these results as the fact that BM could be either a sign of a more aggressive disease or a final result of a different pathogenesis; tumors located in the rectal site have a higher incidence of lung metastases due to differences in the vascular anatomy that probably explain this disparity in metastatic pattern if compared to upper colon cancer.
We have reported a median OS of 48 months from the diagnosis of primary CRC and a median survival of 5 months from the diagnosis of BM. In spite of this relatively long median OS, (better than OS reported in the range from 28.4 to 38.6 months by other studies, 2–3.7), survival after brain metastasization descends sharply into percentages below 10 % similar to literature data [1–4, 7]. The BM free interval was 36 months either in our series or in other studies [1–3]. In our retrospective study, Dukes’ stage at first diagnosis of colorectal cancer is the only factor significantly related with BM free interval, and this was reported in literature data. [1]. Adjuvant therapy and amount of chemotherapy before BM (<1 versus ≥2) have no statistically significant relation to BM free interval even if patients who received more than one line of chemotherapy have a longer median BM free interval than those who received less than one.
Dukes’ stage and type of BM (synchronous versus metachronous) are associated to better outcome at univariate analysis while no impact on OS was found to be related to gender, age, tumor grading, and number and location of brain metastases. When we have tried to correlate patients’ age at BM diagnosis, other metastatic sites involvement or not and BM-related symptoms or not with OS, we have found that only the presence of symptomatic BM seems to be a worse prognostic factors even if the small sample size does not allow definitive conclusions. In Wronski’s review [3], no impact on OS was found to be related to gender, number of metastases, presence of synchronous lung localizations, and the use of adjuvant radiotherapy after metastasectomy, while the cerebellar localization seemed to be a negative prognostic factor.
Jung and coauthors [6] sustained that amount of chemotherapy before BM is an independent prognostic factors for survival; patients with metastatic disease concomitant to BM have a longer survival rate after BM diagnosis than those who developed brain metastases after the diagnosis of metastatic colorectal cancer. However, whole survival after diagnosis of metastatic colorectal cancer was significantly longer in patients with greater than the 12-month interval between metastatic colorectal cancer diagnosis and BM.
In our trial, no impact on survival after BM was found to be related to timing of BM diagnosis and number of BM lesions (Table 5). No impact on BM free survival was found for amount of chemotherapy before BM diagnosis in our study.
Recently, a literature review of various treatment plans and outcome for BM from colorectal cancer reported that a patient who received gamma knife radiosurgery (GSK) plus whole brain radiotherapy (WBRT) or craniotomy have better median OS (10 and 9 months, respectively) than those who received GSK alone (6 months) or WBRT alone (3 months); the increased amount of radiation delivery to BM using WBRT plus GSK may be the reason for the increased survival in patients receiving both the latter regimens . In this review, no data regarding chemotherapy was available [8].
A retrospective study [3] evaluated the outcome of 73 patients undergoing surgery for brain metastases from colorectal cancer between 1974 and 1993; surgery remains the most efficacious and expeditious course to be taken in a large, single, or double accessible BM in a patient who is symptomatic and in whom limited systemic disease is under control, allowing a survival of 6 months or more. WBRT after craniotomy had no impact on survival in this study and was not associated with a decrease in the intracranial recurrence rate.
Other reports have showed a better survival after BM diagnosis in patients underwent surgery than radiotherapy. In all of them, the surgery seems to be the best choice in presence of single BM, asymptomatic patients, and lower extracranial disease involvement [1, 2, 9].
In another study [10], it was reported that patients who received chemotherapy after being diagnosed with BM had a better prognosis than those who did not receive systemic treatment (12.4 versus 3.1 months, respectively), and 40 % of them had been treated previously with the chemotherapeutic agents fluropyrimidine, oxaliplatin, and irinotecan. In another small study (only 12 patients), the longest survival was observed in a patient who survived for 505 days after the start of oxaliplatin and fluorouracil plus radiation therapy [11].
Our results showed that patients who received surgery plus radiotherapy or chemotherapy after diagnosis of BM have better survival after BM than those who received radiotherapy plus chemotherapy or radiotherapy alone or best supportive care alone (Table 5). When we compared these data in multivariate analyses, the combined treatment (surgery ± chemotherapy ± radiotherapy) results significantly better than best supportive care and radiotherapy alone; overall survival after BM diagnosis is longer with combined treatment, probably due to small sample size.
The results regarding the impact on survival of type of intervention for brain metastasis seem to demonstrate that surgery have an important role in improving survival mainly when associated to radiotherapy or chemotherapy; probably, it may be explained by the amount of systemic spread. In detail, in our study, majority (95 %) of patients have an extracranial involvement and 34 % multiple brain lesions.
We reasonably hypothesized that in the presence of single metastasis, the best approach could be surgery or stereotactic radiotherapy, while in the presence of multiple metastases, WBRT remains the cornerstone treatment except in case of lesions suitable to radical resection where surgery followed by radiation therapy could be considered as a viable option. It has to be evaluated the role of chemotherapy, especially in patients with worse prognostic factors.
Recently, biological factors, such as the chemokines’ receptors CXCR4 and the mutational status of KRAS gene, have been evaluating in BM cancer cells with the aim to characterize a specific phenotype [12]. KRAS gene has been found to be more frequently mutated in patients with lung and brain (62 and 56.5 %, respectively) than the ones with liver localizations (32 %) [13]. In our series, we have found that KRAS is mutated in the majority of the patients, but a statistically significant correlation to outcome is not observed due to the small sample size. An interesting tool is to evaluate if the presence of KRAS mutation could be a negative prognostic factor for patients at risk of brain relapsed and suitable for more aggressive treatment or closer follow-up. A main objective for the future researches should be to assess the response and outcome of patients with brain metastases treated in clinical trials with biological agents implicated in EGFR or angiogenesis pathways inhibition.
Prognosis of BM is still poor (median 5 months). But it is too noteworthy that in our trial, overall survival after initial diagnosis of CRC was 48 months with median disease free interval between primary tumor and BM of 36 months. So, BM tends to occur late and under various circumstances. So, it is important to identify molecular and clinical prognostic factors, as lung or intrathoracic lymph node involvements that could be associated with potentially higher risk of BM and eventually to tailor follow-up with the aim to improve BM detection at earlier stage suitable to surgery approach and better outcome.
References
Hammoud MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ (1996) Colorectal carcinoma and brain metastasis: distribution, treatment and survival. Ann Surg Oncol 5:453–463
Alden TD, Gianino JW, Saclarides TJ (1996) Brain metastases from colorectal cancer. Dis Colon Rectum 39(5):541–545
Wronski M, Arbit E (1999) Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer 85(8):1677–1685
Nieder C, Pawinski A, Balteskard L (2009) Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology 76:369–374
Gaspar L, Scott C, Rotman M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Rad Oncol Biol Phys 37:745–751
Jung M, Ahn JB, Chang JH et al (2011) Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol 101:49–55
Krueser TJ, Chao ST, Elson P et al (2008) Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer 113(1):158–165
Morovic JA, Chang SD (2011) Literature review of various treatment plans and outcomes for Brain Metastases from colorectal cancer. World Neurosurg 79(3–4):435–436
Farnell GF, Buckner JC, Cascino TL, O’Connell MJ, Schomberg PJ, Suman V (1996) Brain metastases from colorectal carcinoma. The long term survivors. Cancer 78:711–716
Baek JY, Kang MH, Hong YS et al (2011) Characteristics and prognosis of patients with colorectal cancer-associated brain metastases in the era of modern systemic chemotherapy. J Neurooncol 104(3):745–753
Tajima Y, Ishibashi K, Ishiguro T et al (2009) Analysis of 12 cases of brain metastasis from colorectal cancer. Gan No Rinsho 36(12):2245–2247
Mongan JP, Fadul CE, Cole BF et al (2009) Brain metastases from colorectal cancer: risk factors, incidence, and the possible roles of chemokines. Clin Colorectal Cancer 8(2):100–105
Tie J, Lipton L, Desai J et al (2011) KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res 17:1122–1130
Acknowledgments
We thank Dr. Laura Adamoli for the data management.
Conflict of interest
None declared
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magni, E., Santoro, L., Ravenda, P.S. et al. Brain metastases from colorectal cancer: main clinical factors conditioning outcome. Int J Colorectal Dis 29, 201–208 (2014). https://doi.org/10.1007/s00384-013-1781-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1781-y