Abstract
Purpose
The aim of this study was to investigate the efficacy and safety of neoadjuvant cetuximab, capecitabine, and radiotherapy for patients with locally advanced rectal cancer.
Methods
Sixty-three eligible patients were selectively enrolled in this study. Neoadjuvant treatment consisted of cetuximab and capecitabine for 6 weeks and radiotherapy for 5 weeks. Surgical resection was performed 6–8 weeks after the completion of neoadjuvant treatment. KRAS mutation statuses were analyzed retrospectively after the cetuximab treatment. All the patients underwent a standardized postoperative follow-up for at least 3 years.
Results
A pathological complete response (pCR) was achieved in eight patients (12.7 %). Overall down-staging was found in 49 patients (77.8 %). The 3-year disease-free survival (DFS) rate and overall survival (OS) rate was 76.2 % and 81.0 %, respectively. The most common adverse events during neoadjuvant treatment were acneiform skin rash (82.5 %), radiodermatitis (46.0 %), and diarrhea (36.5 %). KRAS mutations were detected in 19 of 63 (31.2 %) tumors. The down-staging rate in patients with KRAS wild-type (WT) was significantly higher than patients with KRAS mutation (P = 0.020). There was no significant difference in the pCR rate, 3-year DFS rate or 3-year OS rate between KRAS WT patients and KRAS-mutated patients.
Conclusion
Neoadjuvant treatment with cetuximab and capecitabine-based chemoradiotherapy is safe and well tolerated. The pCR rate, 3-year DFS rate and OS rate are not superior to the rate of neoadjuvant chemoradiotherapy using two or more cytotoxic agents. The KRAS WT is highly associated with tumor down-staging to cetuximab plus capecitabine-based CRT in patients with LARC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer persists as a significant worldwide problem [1].The crude incidence of rectal cancer was 11.72 per 100,000 population in China reported by national office of China for cancer prevention and control [2]. Overall mortality at 5 years is about 40 %. Rectal cancer is commonly diagnosed at a precocious stage, but because of local recurrence and distant metastasis, only half of radically resected patients can be considered disease-free [3, 4].The use of a multidisciplinary approach, which integrates surgery, chemotherapy and radiotherapy, has become of increasing importance in rectal cancer. 5-fluorouracil (5-FU)-based chemoradiotherapy (CRT) followed by total mesorectal excision has become the standard of care for patients with locally advanced rectal cancer (LARC), especially in tumors of the lower and middle rectum [5, 6]. In previous studies, preoperative 5-FU-based CRT has been shown to improve pathologic complete response (pCR), tumor down-staging [7] and locoregional control [8, 9] in patients with LARC. Although continuous infusion 5-FU has a biologic advantage of prolonging 5-FU exposure to tumor cells and improving antitumor activity, there are potential problems associated with central venous catheters, such as infection and deep vein thrombosis [10].
Capecitabine, an oral fluoropyrimidine carbamate, provides a more convenient alternative to 5-FU continuous infusion. Several retrospective and prospective trials suggested that preoperative capecitabine had been at least equivalent to infusional 5-FU when combined with radiotherapy, and may improve tumor down-staging [11–15]. It has been reported in recent phase II clinical trials that preoperative capecitabine-based CRT achieves encouraging down-staging and sphincter preservation while exhibiting a low toxicity profile; medium- or long-term survival data were not assessed [11–15]. Survival results of infusional 5-FU and preoperative radiation have been reported in previous randomized phase III trials. The addition of infusional 5-FU to preoperative radiation did not extend disease-free survival (DFS) or overall survival (OS) compared to radiotherapy alone, although the pCR rate increased and local control rate exhibited improvement [7–9]. Novel chemotherapeutic agents used in combination with capecitabine are currently under investigation in order to improve the DFS and OS of patients with rectal cancer; cetuximab is one of these agents.
Cetuximab is a recombinant human/mouse chimeric epidermal growth factor receptor (EGFR) monoclonal antibody. In a phase III study, it was reported that cetuximab in combination with radical radiotherapy significantly improved overall survival compared to radiation alone in patients with locally advanced head and neck cancer [8]. Moreover, cetuximab has demonstrated considerable activity both as a monotherapy and in combination with chemotherapy in the treatment of colorectal cancer [16, 17]. In retrospective analyses, patients with EGFR expressing rectal cancer undergoing neoadjuvant radiation therapy had a significantly inferior DFS and lower pCR [18]. Based on the positive data in LARC and synergy with radiation therapy, there is a strong rationale to combine cetuximab with neoadjuvant CRT in rectal cancer.
In this study, we investigated the efficacy and toxicity of cetuximab in combination with capecitabine-based CRT in the neoadjuvant treatment of patients with resectable rectal cancer.
Material and methods
Study design
This was an open-label, prospective phase II trial and the protocol was reviewed and approved by the local institutional review board. This study was performed according to the Declaration of Helsinki. All of the patients enrolled in this study provided written informed consent. The primary endpoint was the pCR rate. The secondary outcomes were the overall down-staging rate, toxicity, 3 year OS, DFS, and local control rate. The KRAS gene types were analyzed retrospectively after the treatment of cetuximab.
Eligibility criteria
Patients were eligible if they presented with histologically confirmed stage T3 or resectable (surgery deemed to achieve a R0 or R1 resection) T4 rectal adenocarcinoma and with no evidence of distant metastases. The patients were a minimum of 18 years of age with a World Health Organization (WHO) performance status ≤2. Exclusion criteria included a history of prior chemotherapy, pelvic radiotherapy, known hypersensitivity to capecitabine or cetuximab, active infectious disease, active cancer of another type, and pregnancy.
Examination and treatment
Prior to study initiation, all eligible patients underwent a complete medical history, a physical examination, rectoscopy biopsy for the confirmation of adenocarcinoma, colonoscopy, endoscopic ultrasound, digital rectal examination, abdominal-pelvis computed tomography scan, magnetic resonance imaging, chest X-ray, liver ultrasound, and measurement of tumor serum markers CEA and Ca 19-9, complete blood cell counts, blood electrolytes, serum creatinine and urea, blood glucose, calcium, liver AST, alkaline phosphatase, total bilirubin, prothrombin time, partial thromboplastin time, fibrinogen, and cardiological evaluation with ECG.
A summary of the study treatment schema is shown in Fig. 1. Patients received 400 mg/m2 cetuximab as a loading dose in the first week, followed by 250 mg/m2 weekly in conjunction with radiotherapy. Capecitabine was taken orally 1,250 mg/m2 twice daily as a loading dose in the first week, and 850 mg/m2 twice daily for the duration of radiotherapy. Capecitabine was taken within 30 min after breakfast and dinner 12 h apart. Cetuximab infusion was conducted 2 h prior to radiotherapy. The doses of capecitabine and cetuximab that we administered were in accordance with the recommendations from previous phase I dose tolerance studies [19–21]. Radiation of 45 Gy was delivered in 25 fractions with a linear accelerator (energy range 6–18 MV), at 1.8 Gy per day, 5 day per week in accordance with the protocol of the European Organization for Research and Treatment of Cancer (EORTC) 22921 trial.
During the course of treatment, clinical evaluation and complete blood count were performed weekly. Toxicities were graded using the WHO common criteria. Dose modification was determined in accordance with the greatest toxicity. Re-evaluation of the primary tumor was performed with pelvic MRI and endoscopic ultrasound. The re-evaluation and the assessment of tumor response were defined using the Response Evaluation Criteria in Solid Tumors criteria.
All patients underwent surgical excision within 6–8 weeks after completion of neoadjuvant therapy. The decision regarding temporary stoma during surgery was left to the surgeon’s discretion. Administration of adjuvant chemotherapy was left to the treating physician’s discretion. The recommended standard surgical procedure was rectal surgery with total mesorectal excision. Postoperative, pathological evaluation of the surgical specimens was performed. Pathological complete response was defined as the complete disappearance of all tumor cells.
DNA extraction and mutation analysis
We searched for KRAS point mutations in codons 12 and 13. DNA was extracted from paraffin-embedded tumor tissue samples using QIAmp DNA Mini Kit (Qiagen, Shanghai, China), after a histologic control of the presence of tumor cells (>70 %) in each tumor sample by haematoxylin–eosin-stained coloration. The sequences of primers used for KRAS analysis were identical to those used in a previous study [22]. Sequencing reactions were run on the ABI PRISM-310 Genetic Analyser (Applied Biosystems, Peking, China). Sense and antisense strands were analyzed with the Sequence Navigator Software (Applied Biosystems, Peking, China).
Follow-up and evaluation
Patients underwent standardized postoperative follow-up for a median period of 39 months (range, 37–44 months) involving physical examination including a digital rectal examination, complete blood count, liver function test, and serum carcinoembryonic antigen test every 3 months for the first postoperative year and every 6 months thereafter. CT scanning from the chest to the pelvis and colonoscopy were performed every 6 months during the first year and annually thereafter. Relapse was diagnosed pathologically by surgical resection, biopsy, cytology, and/or radiologic findings demonstrating an increase in tumor size over time. Local recurrence was defined as evidence of tumor within the pelvic or perineal area or at the site of anastomosis. Distant metastasis was defined as any relapse outside of the pelvic cavity. OS was defined as the time from study entry to death from any cause. DFS was defined as the time from randomization to local, regional, or distant treatment failure; other second primary cancer; or death without evidence of rectal or second primary cancer.
Statistical analysis
At the time of the study design, the pCR rate for neoadjuvant capecitabine-based CRT in LARC ranged from 4 to 31 % reported by previous phase II/III trials [3, 11, 15]. This study aimed to evaluate whether 18 % pCR could be achieved using this treatment approach. Setting 4 % as the lowest pCR rate of interest, and with an alpha error of 5 % and a power of 80 %, we determined that at least 60 evaluable patients were needed for this evaluation. Differences in pCR rates, down-staging rates and 3-year local control rates between patients with and without KRAS mutations were evaluated by means of a two-sided Fisher’s exact test. A P value equal or <0.05 was considered to indicate statistical significance. The DFS and OS analyses of all the patients were determined according to the Kaplan–Meier method and survival curves were compared using the log-rank test between patients with and without KRAS mutations. Statistical analysis was performed using the SPSS statistical software package, version 16.
Results
Patient characteristics
Between September 2007 and March 2008, 63 patients with T3 or T4 and N0-2 rectal cancer were selectively enrolled in this study. A summary of patient demographic characteristics and tumor status is shown in Table 1. This study included 24 women and 39 men. The median age was 64 years (SD = 6.0 range = 50–77 years). The most frequent tumor stage was T3N1–2 as evaluated by endoscopic ultrasound and MRI. TNM staging at baseline was: T3N0, 12.7 % (8/63); T3N1, 33.3 % (21/63); T3N2, 41.2 % (26/63); T4N0, 3.2 % (2/63); T4N1, 3.2 % (2/63) and T4N2 6.3 % (4/63). The median tumor size was 28 (range 8–45) mm. All tumors were mid- or low rectal cancer, with a median distance from anal verge of 5 (range 1–9) cm.
Toxicities of neoadjuvant therapy and surgery
A summary of the toxicities that occurred during neoadjuvant treatment is presented in Table 2. In total, the most common adverse events during neoadjuvant treatment were acneiform skin rash (82.5 %, 52/63), radiodermatitis (46.0 %, 29/63), and diarrhea (36.5 %, 23/63). The most frequent grade 1–2 toxicity was acneiform skin rash (76.2 %, 48/63). The most frequent grade 3 side-effect was radiodermatitis (15.9 %, 10/63). No grade 4 toxicity was observed. Cetuximab was suspended for one week in four patients due to grade 3 acneiform rash, and treatment subsequently continued with a dose of 200 mg/m2. Grade 3 diarrhea was seen in four patients. After fluid infusion and treatment with loperamide the symptoms of diarrhea subsided. All patients completed the 5 weeks of neoadjuvant therapy of cetuximab, capecitabine, and radiotherapy. Postoperative complications occurred in ten patients, including four patients with anastomotic leakage and six patients with wound infection.
Efficacy
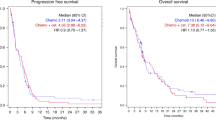
In this study, pCR was observed in eight patients. The pCR rate was 12.7 %. Overall down-staging was found in 49 patients (77.8 %) as shown in Table 3. T level and N level down-staging were found in 35 (55.6 %) and 39 (61.9 %) patients, respectively. Surgical excisions were performed in all patients within 6–8 weeks after completion of neoadjuvant therapy. The anterior resection, low anterior resection, and abdominoperineal resection were performed in 15, 29, and 19 patients, respectively. The total sphincter preservation rate was 73.0 % (46/63). The median follow-up period for all enrolled patients was 39 months (range, 37–43 months). No patient was lost to follow-up. Recurrence occurred in ten patients, including pelvic relapse in four patients and distant metastases in six patients. The 3-year local control rate was 79.2 % (95 % CI: 0.692–0.892). DFS rate was 76.2 % (95 % CI: 0.656–0.867; Fig. 2). Two patients with pelvic relapse were treated with local excision followed by chemotherapy and radiation; the other two patients subsequently underwent abdominoperineal resection. Patients with distant metastases composed of five patients exhibiting liver metastasis and one patient exhibiting lung metastasis. Patients with distant metastases were treated with salvage surgery followed by chemotherapy. As of May 2011, 12 patients (19.0 %) of the entire study population have died. Seven (11.1 %) died of rectal cancer, one patient (1.6 %) died of a second primary cancer and the remaining four patients of other causes (6.3 %). The 3-year overall survival rate was 81.0 % (95 % CI: 71.3–90.6) (Fig. 3).
KRAS analysis
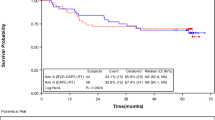
KRAS mutations were detected in 19 of 63 (31.2 %) tumors. The tumor response and survival data of patients with and without KRAS mutation were listed in Table 4. In pCR patients, KRAS mutation and KRAS wild-type (WT) were present in two (25 %) and six (75 %) cases, respectively. pCR rate in patients with KRAS WT (6/44, 13.6 %) patients were higher than patients with KRAS mutation (2/19, 10.5 %), but the difference did not reach statistical significance (Fisher’s exact test, P = 1.000). In down-staging patients, KRAS mutation and KRAS WT were present in 11 (57.9 %) and 38 (86.4 %), respectively. The down-staging rate in patients with KRAS WT (38/44, 86.4 %) was significantly higher than patients with KRAS mutation (11/19, 57.9 %; Fisher’s exact test, P = 0.020).
Survival analysis showed that the 3-year DFS in KRAS WT patients was 76.7 % (95 % CI 64.16–89.24 %) compared with 75.0 %in KRAS-mutated patients (95 % CI 55.99–94.01 %; log-rank, P = 0.888). The 3-year OS in KRAS WT patients was 81.4 % (95 % CI 69.84–92.96 %) versus 80.0 % in KRAS-mutated patients (95 % CI 62.56–97.44 %), but the difference did not reach statistical significance (log-rank, P = 0.870).
Discussion
Presently, neoadjuvant chemoradiation is widely accepted as the standard of care for locally advanced rectal cancer with 5-FU or capecitabine as the single chemotherapeutic agent. Cetuximab and capecitabine in combination with radiotherapy serve as a novel neoadjuvant therapy strategy for rectal cancer. In the present study, this neoadjuvant treatment strategy led to a pCR rate of 12.7 % and down-staging rate of 77.8 % in patients with LARC. This result is consistent with a recent review of pooled data that demonstrated an overall pCR of 9.1 % for cetuximab-based CRT [23]. However, this pCR rate in the present study is still not superior to the pCR rate of 13–32 % reported in recent phase II trials of neoadjuvant CRT that use two or more cytotoxic agents [18, 23–26]. Cell cycle effects seem crucial to achieve these results, because cetuximab can lead to up-regulation of the cyclin-dependent kinase inhibitor p27 and induce the arrest of the cell cycles in the G1 phase. If only a small proportion of cells within the tumor are affected by CRT, this decrease in proliferation could impact on the chance of achieving a pCR [23]. Several mechanisms may also contribute to the apparently subadditive interaction between RCT and cetuximab, including the redundancy of EGFR pathways and sequence dependencies [26, 27].
In the present study, the 3-year local control rate was 79.2 % (50/63). The 3-year DFS and 3-year OS were 76.2 % and 81.0 %, respectively. To the authors’ knowledge, there were no phase III or phase II trials to confirm the survival results with a similar protocol. The present study provides the first survival data of neoadjuvant treatment consisted of cetuximab and capecitabine-based CRT for patients with LARC. Some phase II studies of neoadjuvant capecitabine-based CRT, or capecitabine-based CRT with irinotecan or oxaliplatin that have been conducted reported 3-year DFS and OS of 60–83 % and 68–90 %, respectively [9, 28, 29]. All of these studies were phase II studies with 21 to 31 patients enrolled [9, 28, 29]. To assess the value of EGFR-targeted agents in this setting, future randomized, controlled trials with lager sample size are warranted.
The safety profiles of capecitabine and cetuximab were favorable. The treatment-related toxicity was manageable in most patients. The most common adverse events included acneiform skin rash (82.5 %, 52/63), radiodermatitis (46.0 %, 29/63), and diarrhea (36.5 %, 23/63). The doses of cetuximab and capecitabine were selected in accordance with the results of previous dose-finding studies [28, 30, 31]. Dunst et al. identified the maximum tolerated dose of continuous capecitabine as 1,000 mg/m2 twice daily [19]. The recommended dose for phase II studies was capecitabine 825 mg/m2 twice daily every day plus standard radiotherapy [19–21]. In this study, we used the dose of 825 mg/m2.
Recently, data from an increasing number of studies have suggested that response to cetuximab is confined to patients suffering from rectal cancer with KRAS WT [12, 29, 32]. The KRAS gene, as a predictive and prognostic factor, is needed to identify the subpopulation of patients who truly benefit from cetuximab [33–36]. In this study, we also found that the down-staging rate of patients with KRAS WT was significantly higher compared with the down-staging rate of KRAS-mutated patients. However, we did not observe a significant difference in pCR rate, DFS or OS between patients with KRAS mutation and KRAS WT. This may be due to the impact of capecitabine or surgical resection. Furthermore, our sample size is not sufficiently large enough to detect modest differences between patients with KRAS mutation and KRAS WT. Future prospective studies with lager sample size aimed to assess correlations between KRAS status and neoadjuvant cetuximab in LARC patients will therefore be necessary in order to extend these findings.
Conclusion
Neoadjuvant treatment with cetuximab and capecitabine-based CRT is safe and well tolerated. The pCR rate exhibited in this study is not superior to the pCR rate of neoadjuvant CRT that use two or more cytotoxic agents. The 3-year DFS and OS were similar to the previous 5-FU based neoadjuvant CRT. KRAS WT is highly associated with tumor down-staging to cetuximab plus capecitabine-based CRT in patients with LARC.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics, 2006. CA Cancer J Clin 56(2):106–130
Yang L, Parkin DM, Ferlay J, Li L, Chen Y (2005) Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev 14(1):243–250
Pasetto LM, Pucciarelli S, Agostini M, Rossi E, Monfardini S (2004) Neoadjuvant treatment for locally advanced rectal carcinoma. Crit Rev Oncol Hematol 52(1):61–71
Dresen RC, Gosens MJ, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, van den Brule AJ, van den Berg HA, Rutten HJ (2008) Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 15(7):1937–1947
Wong RK, Tandan V, De Silva S, Figueredo A (2007) Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev 2:CD002102
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer Group (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345(9):638–646
Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A, Collette L (2005) Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol 23(24):5620–5627
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921 (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24(28):4620–4625
Beckers MM, Ruven HJ, Seldenrijk CA, Prins MH, Biesma DH (2010) Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb Res 125(4):318–321
Dupuis O, Vie B, Lledo G, Hennequin C, Noirclerc M, Bennamoun M, Jacob JH (2007) Preoperative treatment combining capecitabine with radiation therapy in rectal cancer: a GERCOR Phase II Study. Oncology 73(3–4):169–176
Velenik V, Oblak I, Anderluh F (2010) Long-term results from a randomized phase II trial of neoadjuvant combined-modality therapy for locally advanced rectal cancer. Radiat Oncol 5:88
Carlomagno C, Farella A, Bucci L, D’Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano A, Solla R, D’Armiento MR, De Placido S (2009) Neo-adjuvant treatment of rectal cancer with capecitabine and oxaliplatin in combination with radiotherapy: a phase II study. Ann Oncol 20(5):906–912
Fakih MG, Bullarddunn K, Yang GY, Pendyala L, Toth K, Andrews C, Rustum YM, Ross ME, Levea C, Puthillath A, Park YM, Rajput A (2008) Phase II study of weekly intravenous oxaliplatin combined with oral daily capecitabine and radiotherapy with biologic correlates in neoadjuvant treatment of rectal adenocarcinoma. Int J Radiat Oncol Biol Phys 72(3):650–657
Craven I, Crellin A, Cooper R, Melcher A, Byrne P, Sebag-Montefiore D (2007) Preoperative radiotherapy combined with 5 days per week capecitabine chemotherapy in locally advanced rectal cancer. Br J Cancer 97(10):1333–1337
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majó J, Benavente S, Armengol M, de Torres I, Grupo Español de Investigacion Clinica en Oncologia Radioterápica (GICOR) (2005) The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol 74(2):101–108
Machiels JP, Sempoux C, Scalliet P, Coche JC, Humblet Y, Van Cutsem E, Kerger J, Canon JL, Peeters M, Aydin S, Laurent S, Kartheuser A, Coster B, Roels S, Daisne JF, Honhon B, Duck L, Kirkove C, Bonny MA, Haustermans K (2007) Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol 18(4):738–744
Hofheinz RD, Horisberger K, Woernle C, Wenz F, Kraus-Tiefenbacher U, Kähler G, Dinter D, Grobholz R, Heeger S, Post S, Hochhaus A, Willeke F (2006) Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. Int J Radiat Oncol Biol Phys 66(5):1384–1390
Hong TS, Kachnic LA (2007) Preoperative chemoradiotherapy in the management of localized rectal cancer: the new standard. Gastrointest Cancer Res 1(2):49–56
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765
Glynne-Jones R, Mawdsley S, Harrison M (2010) Cetuximab and chemoradiation for rectal cancer–is the water getting muddy? Acta Oncol 49(3):278–286
Koeberle D, Burkhard R, von Moos R, Winterhalder R, Hess V, Heitzmann F, Ruhstaller T, Terraciano L, Neuweiler J, Bieri G, Rust C, Toepfer M (2008) Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer 98(7):1204–1209
Glynne-Jones R, Falk S, Maughan TS, Meadows HM, Sebag-Montefiore D (2007) A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study. Br J Cancer 551–558
Weiss C, Arnold D, Dellas K, Liersch T, Hipp M, Fietkau R, Sauer R, Hinke A, Rödel C (2010) Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: a pooled analysis of three prospective phase I–II trials. Int J Radiat Oncol Biol Phys 78(2):472–478
Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, De Neve W (2006) Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys 65(4):1129–1142
Velenik V, Ocvirk J, Oblak I, Anderluh F (2010) A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. Eur J Surg Oncol 36(3):244–250
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 8688–8696
Horisberger K, Treschl A, Mai S, Barreto-Miranda M, Kienle P, Ströbel P, Erben P, Woernle C, Dinter D, Kähler G, Hochhaus A, Post S, Willeke F, Wenz F, Hofheinz RD, MARGIT (Mannheimer Arbeitsgruppe für Gastrointestinale Tumoren) (2009) Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int J Radiat Oncol Biol Phys 74(5):1487–1493
Bertolini F, Chiara S, Bengala C, Antognoni P, Dealis C, Zironi S, Malavasi N, Scolaro T, Depenni R, Jovic G, Sonaglio C, Rossi A, Luppi G, Conte PF (2009) Neoadjuvant treatment with single-agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy: a phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 73(2):466–472
Eriksen JG, Steiniche T, Overgaard J, Danish Head and Neck Cancer study group (DAHANCA) (2005) The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7 study. Radiother Oncol 74(2):93–100
Baker JB, Dutta D, Watson D, Maddala T, Munneke BM, Shak S, Rowinsky EK, Xu LA, Harbison CT, Clark EA, Mauro DJ, Khambata-Ford S (2011) Tumour gene expression predicts response to cetuximab in patients with KRAS wild-type metastatic colorectal cancer. Br J Cancer 104(3):488–495
Zhang W, Labonte MJ, Lenz HJ (2011) KRAS let-7 LCS6 SNP predicts cetuximab efficacy in KRASwt metastatic colorectal cancer patients: does treatment combination partner matter? Ann Oncol 22(2):484–485
Grimminger PP, Danenberg P, Dellas K, Arnold D, Rödel C, Machiels JP, Haustermans K, Debucquoy A, Velenik V, Sempoux C, Bracko M, Hölscher AH, Semrau R, Yang D, Danenberg K, Lenz HJ, Vallböhmer D (2011) Biomarkers for cetuximab-based neoadjuvant radiochemotherapy in locally advanced rectal cancer. Clin Cancer Res 17(10):3469–3477
Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019
Role of the funding sources
There is no involvement of funding sources.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, PL., Li, B. & Ye, QF. Effect of neoadjuvant cetuximab, capecitabine, and radiotherapy for locally advanced rectal cancer: results of a phase II study. Int J Colorectal Dis 27, 1325–1332 (2012). https://doi.org/10.1007/s00384-012-1446-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1446-2