Abstract
Purpose
Early detection and multidisciplinary treatment of colorectal liver metastases (CLM), preferably resection, can significantly prolong the survival of colorectal cancer patients. The purpose of this study was to analyze the incidence, management and long-term clinical outcome of CLM patients using data from a regional German tumour registry.
Methods
We conducted a retrospective analysis of 884 patients diagnosed with colorectal adenocarcinoma in the year 2002 and documented in a regional tumor registry in Southern Germany.
Results
Two hundred thirty-six patients (26.7%) had or developed CLM, 132 patients (14.9%) had synchronous CLM and 104 patients (11.8%) developed metachronous CLM. At diagnosis of CLM, 86 patients (36.4%) had 3 or less documented lesions, 6 patients (2.5%) had 4 to 6 lesions and 89 patients (37.7%) showed multiple, diffuse metastases; for 55 patients (23.3%), the number of lesions was not specified. CLM patients (19.1%) (5.1% of all patients) underwent liver resection; a higher resection rate (28.3%) was observed in a subgroup of patients treated in two academic centres. Patients without CLM had a significantly better 5-year survival rate than patients with liver metastases (65.5% versus 16.3%). CLM patients with up to 3 liver metastases (i.e., potentially resectable) who underwent liver resection (n=34) showed a significantly higher 5-year survival than non-resected (n=52) patients (40% versus 5%).
Conclusions
The present study is the first population-based analysis of the surgical management and outcome of CLM in Southern Germany. The percentage of liver resections was lower than expected, particularly for patients with three or less metastases. The present data suggest that relevant undertreatment of CLM patients may occur which may have a negative impact on survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal adenocarcinoma is the second most common cancer in both men and women in Germany. Approximately 70,000 patients are diagnosed with colorectal cancer (CRC) per year resulting in approximately 28,000 deaths each year [1]. Although 85% of patients diagnosed with CRC can undergo local resection, which is initially curative [2], a leading cause of death is local recurrence and/or metastatic disease. The primary metastatic site for patients with CRC is the liver: 60–70% of metastatic recurrences in CRC patients occur in the liver, and up to 35% of metastatic CRC patients have metastases only in this organ [3]. Approximately 25% of CRC patients present colorectal liver metastases (CLM) synchronously with the primary tumour and 30% develop CLM within 24 months after primary diagnosis [4]. The median survival of metastatic CRC patients can be prolonged from 6 months in untreated patients to 25 months using modern chemotherapeutic regimens [5, 6]. However, a treatment concept including liver surgery remains the only curative approach [7–10].
Surgical management of CLM has undergone a significant change during the last decades. Previously, surgical interventions for CLM were limited to patients with a maximum of three unilobular metastases, of which the largest had a maximum diameter of 5 cm and with no evidence of extrahepatic metastases at the time of surgery [2, 10, 11]. Advances both in multimodality treatment and surgical technique have led to a significant increase in liver resections for CLM. In 2006, an expert consensus recommended liver resection for all patients meeting the following criteria: (1) manageable margin-negative resection, (2) residual liver of ≥2 contiguous hepatic sectors, (3) adequate perfusion and biliary drainage and (4) >20% residual healthy liver [8]. Since then, only a few population-based studies have analyzed the surgical management of CLM in general hospitals and academic centres. The present study is the first of this kind in Southern Germany, performed in Bavaria using data from the Regensburg Cancer Registry, Germany.
Patients and methods
Patient population and data acquisition
The study population included all patients that were diagnosed with colorectal adenocarcinoma in the year 2002 and registered in the Tumorzentrum Regensburg (TUZR, Regensburg Cancer Registry). The TUZR collects epidemiological and clinical information on all cancer patients in the Southern German Regions of Upper Palatinate and Lower Bavaria. These regions have a total population of approximately two million inhabitants. Data were collected from standardized cancer report sheets submitted from care centres and oncologists as well as from archived hospital discharge letters for each patient. All diagnoses were confirmed by histology. Life status of the patients was ascertained using death certificates and information from the registration offices of the patients’ respective resident districts. The observation time was the interval between the diagnosis of primary tumour until last follow-up or death of patient. Cut-off date was February 2007. Patients suffering from more than one tumour entity, e.g. another malignancy in addition to colon cancer, were excluded. The study population includes a total of 884 patients with complete clinical records, representing a completeness of 85%. Data pertaining to demographics, TNM staging, grading, histology, completeness of resection, adjuvant treatment, localization, time and characteristics of metastases, surgical interventions, recurrences, disease-free periods, outcomes and type of treatment institution (academic centre or general hospital) were reviewed for each patient and entered into a database.
Synchronous metastasis was defined as a lesion diagnosed/documented simultaneously or within 3 months of the diagnosis of the primary tumour—under the consideration that a real hepatic tumour “recurrence” that early after primary treatment is very unlikely. A diagnosis of metastases later than 3 months after the primary was defined as metachronous metastasis. All collection of patient information was approved by the Bavarian Law of Cancer Registration.
Statistical analysis
Overall, survival time was censored at the time of death or last follow-up with a cut-off date in February 2007 in order to have a follow-up of 5 years for each patient. Survival curves were estimated by the Kaplan–Meier method. Results were considered significant at p < 0.05. All statistical and descriptive analyses were performed using SPSS software, version 18.0.
Results
Occurrence of synchronous and metachronous liver metastases
Data were obtained for a total of 884 patients who had been diagnosed with CRC in the year 2002 (Fig. 1). Of those, 236 patients (26.7%) were documented to have developed liver metastases during the 5-year follow-up. The timing of occurrence of CLM is shown in Fig. 2. One hundred and thirty-two of those 236 patients (55.9%) had synchronous metastases, which were documented at the time of initial diagnosis in 87 patients (36.8%) and by a month 3 in 45 patients (19.1%). Thus, in 45 out of 132 patients (34.2%), synchronous metastases were recognized (or documented) only within the first 3 months after the primary operation. One hundred four patients (44.1%) developed metachronous CLM (i.e. liver metastases documented beyond 3 months after the diagnosis of the primary tumour). In all cases, the diagnosis of liver metastases was based on ultrasound plus CT, MRI and/or surgical examination during resection of the primary tumour. Of all metastases, 74.1% were diagnosed within 12 months, 85.7% within 24 months and 97.1% within 36 months after the diagnosis of the primary tumour (Fig. 2).
Characteristics of CLM patients and of colorectal liver metastases
Tables 1 and 2 summarize the characteristics of the 236 patients suffering from CLM and compare their demographic and primary tumour staging data with the 648 patients without liver metastasis. Among the CLM patients, 60.6% were male and 39.4% female. The vast majority was older than 51 years (85.5%) with a mean age at diagnosis of 65 (median, 66 years).
At first diagnosis of CLM, a single liver nodule was documented in 57 patients (24.2%), while up to three nodules were documented in 86 patients (36.5%); in 95 patients (40.2%) 4 or more nodules were documented, while no documentation about the number of lesions existed for 55 patients (23.3%). The size of metastases could not reliably be assessed since documentation was missing in 63.2% of the cases. In 23.7% of CLM patients, it was documented that three or less hepatic segments showed metastatic nodules, while in 37.6% disease in four or more segments was described. In 91 patients (38.7%), the distribution of hepatic metastases was not documented.
Surgical approach to hepatic metastases
Of all 236 patients with documented CLM, 45 patients (19.1%) underwent liver resection with curative intent (Table 3) with a larger proportion of 42.1% in patients with single metastasis. The surgical approach included 17 atypical resections, 13 segmental resections, 5 right hemihepatectomies and 10 combination surgeries (i.e. hemihepatectomy and atypical resection, hemihepatectomy and segmental resection or segmental and atypical resection). With regard to the timing of hepatic surgery, the vast majority of patients underwent liver surgery in a separate operation after prior resection of the primary colon cancer. No patient underwent reverse resection (hepatic resection before colectomy) and only two patients underwent synchronous bowel and liver surgery. Surgeries were performed in a total of 30 hospitals including two academic centres. In a subpopulation of 113 CLM patients, treatment of primary tumour, surveillance and (if applicable) liver resections were performed in two academic centres being part of this study, both specialized in and experienced at liver surgery. Of those 113 patients, liver resection was performed in 32 patients (28.3%). Among all 45 patients undergoing liver surgery, R0 resection was achieved in 28 patients (62.2%), R1 resection in 3 patients (6.7%) and R2 resection in 5 patients (11.1%). In nine patients (20%), the resection status was not documented (Fig. 1).
Few patients underwent ablative therapies (three radiofrequency thermoablations in patients not undergoing liver surgery, three radiofrequency ablations, two infrared coagulations, one instillation of ethanol and one cryoablation in patients with recurring liver metastases after liver surgery).
In 27 of the 45 patients (60%) receiving liver resection, additional chemotherapy was documented versus in 113 of the 191 patients (59.2%) with liver metastases that did not undergo resection. No data on this issue were documented in about 30% of the patients.
Clinical outcome
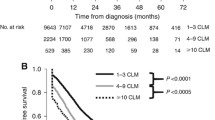
Of all 884 patients, 479 (54.2%) were alive in February 2007. Kaplan–Meier survival curves are shown in Fig. 3. Whereas the 5-year survival was 65.6% for patients without liver metastases, CLM patients showed a significantly lower 5-year survival of 16.3% (p < 0.001). Figure 4 shows the 5-year survival after diagnosis of liver metastasis for CLM patients undergoing liver resection versus CLM patients without liver surgery. Whereas CLM patients undergoing non-surgical management showed a 5-year survival of less than 5.3%, patients undergoing liver resection with curative intent showed a 5-year survival of 38.8% (p < 0.001). Figure 5 depicts the survival after the diagnosis of liver metastasis in relation to the number of metastases documented. There is a significant difference for survival with one and two or three metastases versus more than three metastases (p < 0.001 and p = 0.017). For patients with one metastasis (irrespective of surgical treatment), 5-year survival was 25.8%. Survival for patients with two or three metastases is comparable in the first 2 years, then declines to 4.5% after 5 years (p = 0.216). The survival curve for patients with no documented number of metastases (5-year survival = 10.0%) suggests that these were predominantly patients with multiple metastases. Figure 6 shows survival curves for the 86 patients with up to three liver metastases documented (i.e. potentially resectable), who did (n = 34) or did not (n = 52) undergo liver resection, which was significantly different at 5 years (40.0% versus 5.0%) (p < 0.001). In the subgroup of 57 patients with only one liver metastasis, 5-year survival after the diagnosis of liver metastasis was 50.0% after resection versus 7.6% without resection (p < 0.001)
Comparison of overall survival (Kaplan–Meier) after diagnosis of hepatic metastases in liver resected (solid line) versus unresected (dotted line) patients in 236 patients with colorectal liver metastases. Patients not undergoing surgery showed a significantly lower 5-year survival compared to patients undergoing hepatic resection (5.3% versus 38.8%, p < 0.001). The survival curve of resected patients includes all resected patients independently of their resection margin status
Comparison of overall survival (Kaplan–Meier) after diagnosis of hepatic metastases in relation to the number of metastases documented (one metastasis: n = 57, blue line; two to three metastases, n = 29 green line; >3 metastases, n = 95 red line; and not documented, n = 55, yellow line). Irrespective of surgical treatment, patients with one or two/three liver metastases showed a significantly higher 5-year survival rate than patients with more than three metastases (p < 0.001 and p = 0.017)
Comparison of overall survival (Kaplan–Meier) after diagnosis of hepatic metastases in patients with up to three liver metastases documented (n = 86) that did (n = 34, solid line) or did not (n = 52, dotted line) undergo liver resection. Survival was significantly better in resected patients (40.0% versus 5.0%, p < 0.001)
Discussion
During recent years, remarkable advances in the treatment of CRC and improvement of surgical technique have led to a significant extension of indications for liver resection for CLM patients. Today, liver resection has become accepted as the standard of care for CLM patients with resectable metastases. Single-centre publications have shown 5-year survival rates after liver resection for CLM of up to 50% [7–10]. However, single, high-volume academic centres represent a biased population consisting predominantly of highly selected patients and therefore the results do not reflect the clinical outcome of the average CLM patient. Recently, several population-based studies have analyzed the surgical management and clinical outcome of CLM patients. However, the present study is the first population-based analysis of the incidence, management and clinical outcome of CLM patients in Southern Germany.
In the present study, 236 out of a total of 884 patients diagnosed with CRC in 2002 (26.7%) were documented to have developed CLM within 5 years after the primary diagnosis: about half of them had synchronous metastases and the other half had metachronous metastases. Overall, these findings are at the lower range as compared to other recently published population-based analyses from France, Sweden, Northern Germany, Canada, Great Britain and the USA where CLM rates between 25% and 50% have been observed. Rates of synchronous metastases in these studies using the same definition, however, varied between 13.9% and 71% [9, 12–17]—a fact that probably reflects selection or referral bias of different centres. For our study, it remains unclear whether the rather low rate of over all liver metastases (about 27%) is really true or whether it is due to a documentation deficit—however, the completeness of the registry at about 85% suggests that our data reflect the reality in our region.
In our study population, 45 of the 236 CLM patients (19.1%) underwent liver resection with curative intent. This proportion was higher (over 40%) among patients with single lesions. Single-centre publications have shown that 10% to 20% of CLM are resectable at first diagnosis and up to a further 15% may become resectable after chemotherapy resulting in a higher resection rate than that found in our population [8]. However, the resection rate in our population was higher than all recently published population-based analyses. For instance, a Northern German analysis of 1,299 CLM patients showed a resection rate of 8.8% [12], two French cancer registries published resection rates of 17.3% and 16.9% [13, 17], a Swedish analysis of 537 CLM patients showed a resection rate of 4% [14] and a large US population-based study analyzing 13,599 CLM patients published a resection rate as low as 6.1% [15].
In a British population-based analysis published in 2010, the hepatic resection rate for a population of 114,155 patients diagnosed with CRC [16] (the rate of liver metastases was not specified) was reported to be on average 2.7% for the study period (1998–2004), increasing from 1.7% in 1998 to 3.8% in 2004. In our population, 45 patients underwent hepatic resections among 884 patients diagnosed with CRC, i.e. a rate of 5.1%, which compares favourably to the British data. Further studies would be required in our population to show whether there is also an increase in resection rates over time.
One hundred thirteen of 236 CLM patients (47.9%) in our study were treated at one of two academic centres, while 123 patients were treated at smaller hospitals. Of those 113 patients, liver resection with curative intent was performed on 32 patients (28.3%). Thus, in our population, 32 out of 45 patients (71%) who underwent liver surgery were treated in academic centres. This is comparable to the aforementioned population-based publications. A higher resection rate of patients treated in experienced centres is also consistent with data published from large academic single institution studies and could reflect selection and referral bias, or may also indicate that more patients with resectable CLM actually underwent surgery in these centres. In larger centres, tumour patients are systematically discussed in comprehensive tumour-board meetings, where surgical options for each patient are evaluated on an individual basis by experienced liver surgeons.
In our analysis, CRC patients without liver metastases showed a 5-year survival of 65.6% whereas CLM patients showed an overall 5-year survival of 16.3%. This clearly indicates that patients who develop CLM have a worse prognosis than those who do not. Moreover, the number of metastases was also important in determining the prognosis. Further analyses of CLM patients showed that 5-year survival from the time of diagnosis of liver metastasis was 5.3% for unresected CLM patients and 38.8% for resected CLM patients. These rates are equal to or higher than recent population-based studies, where curatively resected CLM patients had 5-year survival rates between 10.8% and 45% [9, 12–17]. A large academic single-centre analysis of resected CLM patients has recently shown that survival curves for patients undergoing liver resection for CLM reach a plateau at approximately 10 years after hepatic resection [3]. Tomlinson and colleagues have defined “cure” of CLM as 10 years disease-free survival after liver resection and have published cure rates between 17% and 25%. In the same publication, Kaplan–Meier survival analyses showed a 5-year survival of approximately 38%, which is consistent with our data.
Of particular importance is the subset of patients with up to three documented metastases. This represents a subgroup in which the majority of patients would be considered to have resectable liver metastases. From the documentation, it is not clear why a large number of these patients were not treated surgically. Among this subgroup, liver resection was associated with a 5-year survival of 40.0% after resection versus 5.0% without resection. Interestingly, even in the subgroup of patients with only one documented metastasis, more than half of the patients did not undergo resection (58%), and here the difference in 5-year survival was even more profound (50% for the resected group versus 8% without resection). Although some patients with few metastases might not have undergone resection because of other medical contraindications (data that could not be obtained from the registry), it is very plausible that in a relevant proportion of these patients, the option for liver resection might not have been considered and discussed at all. Thus, our data suggest an undertreatment particularly in this subgroup of patients.
A limitation of our study is the lack of systematic documentation of parameters such as resection margin status of the liver as well as details concerning the use of or type and duration of chemotherapy. Therefore, correlations between resection status and outcome and between the use of chemotherapy in patients with or without liver resection were not possible. These issues are clearly of importance, and thus should be included in further studies. Although the current study lacks this information, the central findings reported herein are not diminished.
In conclusion, our study is the first large population-based analysis on the incidence, surgical treatment and outcome of CLM patients in Southern Germany. Here, we show that CLM in our patient cohort were diagnosed (or documented) at a lower rate than expected. Treatment options offered to patients varied according to the institution, and our study clearly demonstrates that population-based analyses of CLM differ from single academic centre reports. Moreover, our results suggest that the undertreatment may have occurred in a relevant number of patients with limited liver metastasis (up to three metastases). Taken together, these findings emphasize the importance of an (experienced) interdisciplinary management of metastatic colorectal cancer in designated centres. The present analysis will serve as a reference for future improvements to multidisciplinary treatment and surveillance of colon cancer patients at our institution. Early and experienced surgical assessment plays a pivotal role for long-term clinical outcome, offering the chance of improved prognosis or cure. Given the obvious survival benefit, the possibility of performing liver resection has to be evaluated individually for every patient with CLM before starting systemic chemotherapy and as soon as possible after diagnosis.
References
Schmiegel W, Pox C, Reinacher-Schick A et al (2008) S3-Leitlinie Kolorektales Karzinom. Z Gastroenterol 46:1–73. doi:10.1055/s-2008-1027700
Donadon M, Ribero D, Morris-Stiff G, Abdalla EK, Vauthey JN (2007) New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res 1:20–27
Tomlinson JS, Jarnagin WR, DeMatteo RP et al (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25(29):4575–4580. doi:10.1200/JCO.2007.11.0833
Scheele J, Stangl R, Altendorf-Hofmann A (1990) Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 77:1241–1246
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Venook A (2005) Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist 10:250–261. doi:10.1634/theoncologist.10-4-250
Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239(6):818–825
Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D (2006) Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 13(10):1271–1280. doi:10.1245/s10434-006-9045-5
Shah SA, Haddad R, Al-Sukhni W et al (2006) Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 202(3):468–475. doi:10.1016/j.jamcollsurg.2005.11.008
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S (2006) Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 13(5):668–676. doi:10.1245/ASO.2006.05.039
Jarnagin WR, Gonen M, Fong Y et al (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236(4):397–406. doi:10.1097/01.SLA.0000029003.66466.B3
Mantke R, Niepmann D, Gastinger I, Lippert H, Koch K, Quehl A (2006) Hepatic resections. Analysis of data from the Tumor Documentation Center in the state of Brandenburg, Germany, focusing on liver metastases of colorectal carcinoma. Chirurg 77(12):1135–1143. doi:10.1007/s00104-006-1247-7
Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G (2006) A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 93(4):465–474. doi:10.1002/bjs.5278
Sjövall A, Järv V, Blomqvist L, Singnomklao T, Cedermark B, Glimelius B, Holm T (2004) The potential for improved outcome in patients with hepatic metastases from colon cancer: a population-based study. Eur J Surg Oncol 30(8):834–841. doi:10.1016/j.ejso.2004.06.010
Cummings LC, Payes JD, Cooper GS (2007) Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer 109(4):718–726. doi:10.1002/cncr.22448
Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, Cottier B, Poston G (2010) Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 97:1110
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244(2):254–259. doi:10.1097/01.sla.0000217629.94941.cf
Acknowledgements
The authors thank Dr. Chloe Milsom for editorial assistance and Prof. Dr. Ferdinand Hofstädter for critical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hackl, C., Gerken, M., Loss, M. et al. A population-based analysis on the rate and surgical management of colorectal liver metastases in Southern Germany. Int J Colorectal Dis 26, 1475–1481 (2011). https://doi.org/10.1007/s00384-011-1278-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1278-5