Abstract
Introduction
Since Kurzawski et al. described an association between the 3020insC NOD2 single nucleotide polymorphism and the risk of colorectal cancer(CRC) in 2004, reports published in the past several years have controversial results regarding the relationship between the development of CRC and NOD2 gene polymorphisms. To clarify the potential role of NOD2 P286S, R702W, G908R, and 3020insC polymorphisms in CRC patients, we have undertaken a systematic review and meta-analysis of published articles.
Materials and methods
Studies reporting on NOD2 polymorphisms and CRC were searched in the PubMed, EMBASE, and the Science Citation Index from the inception of each database to May, 2009. The search strategy included the keywords “CRC”, “colon cancer”, “rectal cancer”, “polymorphism”, and “NOD2/CARD15”.
Result
Eight eligible case-control studies about Caucasians from four countries contributed data on 5,888 subjects (cases: 3,524; controls: 2,364). Compared to the wild genotype, the R702W, G908R, and 3020insC polymorphisms were associated with an increased risk of CRC (odds ratio (OR): 1.59, 1.98, 1.44; 95% confidence interval (CI): 1.09–2.32, 1.14–3.44, 1.13–1.84; P = 0.02, 0.01, 0.003). However, P268S polymorphism did not influence CRC risk (OR: 1.27; CI: 0.32–5.00; P = 0.73).
Conclusions
These findings indicate that NOD2 R702W, G908R, and 3020insC polymorphisms contribute to CRC susceptibility in Caucasians. Meta-analysis of these polymorphisms in NOD2 gene will help determine their role in CRC carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) ranks as the second leading cause of cancer-related mortality in western countries and the third most common malignancy in the world, causing some 500,000 annual deaths worldwide, and recently the prevalence of this carcinoma has been increasing [1–4]. CRC incurs an annual expenditure of more than £300 million in surgical, adjuvant, and palliative treatment [5]. Widely accepted is that cancer is a disease caused by accumulation of mutations in specific gene [6]. CRC is believed to develop slowly via a progressive accumulation of genetic mutations [2], and it has been a model for investigating the molecular genetics of cancer development and progression [7]. The identification of the important CRC-related genes may help facilitate the early diagnosis, prevention, and treatment of CRC [8]. Many researchers have been drawn to study the genetic basis of sporadic CRC. And the study of the underlying molecular genetics and biology associated with the development and progression of CRC has led to the significant treatment advances over the past 10 years [9]. In this article, we are highlighting those genetic polymorphisms which may be regarded to genetic predisposition of CRC.
The nucleotide oligomerization domain 2 (NOD2/CARD15) gene situates at chromosome 16q12 within the inflammatory bowel disease (IBD) 1 region [10–12]. NOD2 gene is characterized by a tripartite structure with a C-terminal sensor domain (leucine-rich repeats, LRRs), a central nucleotide binding and oligomerization (NOD or NACHT) domain, and an N-terminal effector domain (CARD) [13]. It is a cytoplasmic molecule involved in sensing microbial cell wall components and regulating inflammatory processes and apoptosis [14–16]. In the past few years, NOD2 has become known as key regulator of chronic inflammatory conditions [17] and polymorphisms in NOD2 have been associated with increased susceptibility to Crohn’s disease (CD) [10–12], a human chronic IBD. Recently, investigation of the susceptibility loci governed by polymorphic alleles, particularly those of the innate immune response, is growing [18]. It was believed that chronic inflammation favors tumorigenesis by stimulating cell proliferation and angiogenesis and by inducing DNA damage [19–21]. Patients with CD have a higher risk of developing CRC [22], and a meta-analysis has also revealed a significantly increased risk of CRC in CD [23]. Researchers began to be interested in whether hereditary susceptibility genes in CD also played roles in CRC, which caused them to investigate a possible influence of NOD2 on the development of CRC.
Four major NOD2 single nucleotide polymorphisms have been described as genetic risk factors of CD: one background polymorphism P268S/SNP5, two missense mutations R702W/SNP8, G908R/SNP12, and one frameshift mutation 3020insC(1,007 fs)/SNP13 [11, 24]. Since Kurzawski et al. described a potential association between 3020insC and the risk of CRC in 2004, possible association of the NOD2 polymorphisms P268S, R702W, G908R, and 3020insC with CRC has been studied among Polish, Greek, Finnish, Hungarian, and New Zealand Caucasian CRC patients [25–32]. However, results are controversial. To clarify the role of NOD2 polymorphisms in the development of CRC, we undertook a systematic review and meta-analysis of published studies.
Materials and methods
Search strategy and selection criteria
The published Quality of Reporting of Meta-analysis (QUOROM statement) was followed [33]. Studies investigating the relationship between CRC and NOD2 polymorphisms were carried out by searching for articles written in English in PubMed, EMBASE, and the Science Citation Index, and limited our search to English papers, from the inception of each database to May 2009. Various combinations of the terms “CRC”, “colon cancer”, “rectal cancer”, “polymorphism,” and “NOD2/CARD15” were used to screen for potentially relevant studies. Inclusion and exclusion criteria: case-control or cohort studies presenting original data on associations between CRC and NOD2 polymorphisms were included.
Data extraction
For all studies, we extracted the following data from original publications: first author and year of publication; genes and relevant polymorphisms; characteristics of the study design and the study population (numbers of cases and controls, matching criteria, study base and host ethnicity). Two reviewers independently extracted data. Disagreements were resolved by discussion. Authors were contacted for further information when necessary.
Statistical analysis
The strength of the associations between CRC and NOD2 polymorphisms was estimated by odds ratios (OR) and 95% confidence intervals (CI). We addressed the association between the presence of at least one high-risk allele and CRC susceptibility and the effect of each high-risk allele was examined separately.
For the rare frequencies of NOD2 P286S, R702W, G908R, and 3020insC homozygous mutants (+/+), we estimated the risk of the homozygous mutants (+/+) and heterozygous (−/+) versus homozygous wild-types (−/−), respectively.
The software HWE (http://linkage.rockefeller.edu/ott/linkutil.htm) was used to evaluate the deviation from Hardy–Weinberg equilibrium in controls. Heterogeneity, evaluated by the Cochrane Q-test among the studies, was considered significant for P < 0.05. The data were combined using both fixed effects and random effects models. Random effects are more appropriate when heterogeneity is present. The analyses were performed with the computer programs Review Manage, version 5.0 (Oxford, England, UK). All P values are two-sided.
Results
Study characteristics
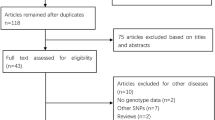
Eighty-five papers relevant to the words searched were retrieved (Fig. 1). Through the step of screening the title, 39 of these articles were excluded (duplicated). Abstracts from 46 articles were reviewed and an additional 20 trials were excluded (clearly not relevant), leaving 26 studies for detailed review. Of these, 18 were excluded (did not meet detailed inclusion criteria); thus, eight papers [25–32], which included 3,524 CRC cases and 2,364 controls, were found to conform to our inclusion criteria. At last, eight case-control studies were included in this meta-analysis. Studies were carried out in Poland, Finland, Greece, New Zealand, and Hungary. A list of details abstracted from the studies included in the meta-analysis is provided through Table 1. The most commonly investigated genotypes were NOD2 P286S, R702W, G908R, and 3020insC, which were reported in two, five, five, and seven studies, respectively. Table 2 showed the allele frequencies and percentage of NOD2 polymorphism carriers among CRC cases and control. Most studies used healthy volunteers or blood donors as control subjects. We assessed deviation from Hardy–Weinberg equilibrium by the HWE program, and the results showed that the genotype distribution of control population in all the eight included studies were in Hardy–Weinberg equilibrium. The publishing year of the included studies ranged from 2004 to 2008. Appropriate molecular methods for genotyping were stated in all studies, all of which was polymerase chain reaction restriction fragment length polymorphism.
Meta-analysis databases
NOD2 P286S polymorphism
Two studies evaluated the NOD2 P286S allele were included in this meta-analysis [29, 31]. Because of the existed heterogeneity (P < 0.05), random model was used. No evidence indicated that individuals carrying the variant genotypes (−/+ · +/+), compared with those carrying the homozygous wild-type (−/−), had an increased risk of CRC (OR 1.27, 95% CI 0.32–5.00, P = 0.73; Fig. 2).
NOD2 R702W polymorphism
The dominant model of the NOD2 R702W allele was conducted in this analysis. The results from five studies [27–29, 31, 32] showed that individuals with the variant genotypes (−/+ ⋅ +/+) had significant risk of CRC compared with those with the homozygous wild-type (−/−; OR 1.59, 95% CI 1.09–2.32, P = 0.02), and there was some heterogeneity among these studies (Fig. 3).
NOD2 G908R polymorphism
We included five studies [27–29, 31, 32] observing the NOD2 G908R allele, the results showed significant difference between individuals carrying the variant genotypes (−/+ ⋅ +/+) with those carrying the homozygous wild-type (−/−; OR 1.98, 95% CI 1.14–3.44, P = 0.01), which suggested that people carrying the variant genotypes of NOD2 G908R had an increased risk of CRC, compared with those carrying the wild-type. No between-study heterogeneity was found in this analysis (Fig. 4).
NOD2 3020insC polymorphism
Seven studies of the NOD2 3020insC were enrolled in this analysis [25–31]. The combined results based on these seven studies showed that, compared with those with the homozygous wild-type (−/−), there was significant risk of CRC of individuals with the variant genotypes (−/+ ⋅ +/+; OR 1.44, 95% CI 1.13–1.84, P = 0.003), and no heterogeneity was indicated (Fig. 5).
People carrying at least one of the variant genotypes
Five of eight studies were eligible for assessing the impact of at least one of NOD2 variant genotypes on the CRC risk [25–28, 30, 31]. Because there was the statistical heterogeneity between studies (P < 0.05), the random effects mode was applied. Among the populations in the included studies, the presence of at least one high-risk allele conferred greater risk for CRC (OR 1.58, 95% CI 103–2.42, P = 0.03; Fig. 6).
Discussion
NOD2 could be an especially important part of innate immunity for maintenance of the intestinal barrier [34], and it is already proved that the alteration in the NOD2 gene participates in the development of IBD. The NOD family of proteins is mainly expressed in monocytes, macrophages, and B cells [35, 36]. In eukaryotes, nuclear factor κB (NF-κB), whose activity is regulated by, among others, NOD2 protein, plays an essential role in the regulation of basic processes of the organism, including immune response, apoptosis, cell cycle control, and the development of individual cell lines [37]. Mutations in the LRRs of the NOD2 gene could disturb NF-κB activation [12, 36]. Due to the crucial involvement of NF-κB in the regulation of cell division mechanisms, it was attributed with an uncertain role in the process of cancer development, where its activity is significantly elevated. Already in the mid-1990s, the importance of NF-κB was described in relation to cancer of the thyroid, breast, lung, and colorectum [38–41]. The loss of NOD2 gene function is predicted to result in excessive NF-κB activity, leading to an inflammation-dysplasia-carcinoma sequence [18]. The literature includes increasing evidence indicating that NOD2 polymorphisms can be considered predisposition factors to malignancy development, such as gastric, bowel, breast cancer [18, 42], and non-Hodgkin’s lymphoma [43].
This is the first meta-analysis of the association of NOD2 polymorphisms with susceptibility to CRC. We quantified distinct risks for CRC of the four common NOD2 polymorphisms, with narrow confidence intervals. On the basis of eight studies, providing case and control numbers of the NOD2 polymorphisms and CRC risk in Caucasians, including 5,888 subjects (cases:3,524; controls:2,364), our meta-analysis provided good evidence that NOD2 R702W, G908R, and 3020insC polymorphisms were associated with increased risk of CRC. P268S, however, was not shown to have impact on CRC risk; and the effect of 3020insC was most significant. Due to the limited studies (two) involved in the association of P268S with CRC, the pooled results may not be of high reliability. More studies are needed to assess the risk impact of NOD2 P268S.
NOD2 polymorphisms have previously been studied in relation to CD susceptibility [10, 11], and it has already been proved that CD patients are susceptibility to CRC [23]. Kurzawski et al. for the first time associated NOD2 polymorphisms with CRC, and drew a conclusion that NOD2 polymorphisms increased CRC risk, from then seven case-control studies involved in this field, and concluded conflicting results [25–32]. Several factors may contribute to the differences among the researches. First, genetic heterogeneity may be a reason for the conflicting results. The frequency of NOD2 polymorphisms vary considerably between races [44]. Such as in Asia, the frequency of NOD2 polymorphisms is very low, and in a large Japanese cohort of CD patients, none of the R702W, G908R, and 3020insC was found [45]. In Australian population, a lower background allele frequency of 3020insC has been observed [46]. Even in European, a low frequency of the NOD2 polymorphisms in the northern countries, compared with the rest of Europe, has been demonstrated [47]. In this meta-analysis, of the included eight studies, there is only one research on evaluating the NOD2 polymorphisms in relation to CRC outside Europe [29], and the contribution of NOD2 polymorphisms to CRC susceptibility varies in different studies.
Second, variation in patient characteristic (e.g., in terms of age and years from onset) might potentially also contribute to the differences among the researches. The results of Kurzawski et al. indicated that NOD2 may be a predisposing factor to CRC characterized by an older average age (>50 years of age) of disease onset in persons [26]. However, Alhopuro et al. concluded contrary results [25]. Some researchers did not consider age onset stratification in their studies. No definite conclusion about the relation between age and CRC risk is available so far. More careful stratification analysis that takes into account the age of onset is needed. However, due to the restricted sample size such differences were not elaborated.
Furthermore, differences in carcinogenic exposure may modify the inherent risk associated with genetic susceptibility [47]. Modifiable risk factors such as physical activity, weight, and diet, just as genetic risk factors have been implicated in the development of CRC [48]. For instance, the average monthly intake of alcohol may have influence on CRC accident [49, 50]. Some research has further provided strong evidence that smoking is associated with an increased risk of CRC [51, 52]. The interaction between NOD2 polymorphisms and environmental carcinogens may represent one of the mechanisms by which NOD2 polymorphisms increase the susceptibility to CRC.
One of the studies involved in our meta-analysis investigated gene–gene interaction. Suchy et al. found the association of TNFα-1,031 T/T genotype and the NOD2 3020insC polymorphisms may act as low risk modifiers of CRC risk [30]. More researches on NOD2 polymorphisms gene–gene interactions will provide a more comprehensive insight into the associations studied here.
Whereas, inherited susceptibility is responsible for about 35% of all CRC [53], high-risk germline mutations account for < 6% of all cases [54]. In addition to the four newly identified loci, so far, there are only ten identified CRC-associated loci: 14q22.2 (rs4444235, BMP4), 16q22.1 (rs9929218, CDH1), 19q13.1(rs10411210, RHPN2), 20p12.3 (rs961253) [55], 8q24 (rs6983267) [56, 57], 8q23.3 (rs16892766, EIF3H), 10p14 (rs10795668) [58], 11q23(rs3802842) [59], 18q21 (rs4939827, SMAD7) [58, 59], and 15q13 (rs4779584) [60]. If NOD2 is another CRC-associated locus, more studies or larger case-control studies about the association of NOD2 polymorphisms and CRC, genome-wide association studies, replication analyses, and cytogenetic tests should be performed. For the study of the underlying molecular genetics and biology associated with the development and progression of CRC will lead to treatment advances, we may benefit from further study on clarifying the possible roles of NOD2 polymorphisms in CRC. Whereas our results found NOD2 polymorphisms seem to confer considerable risk for CRC, most genetic risk factors for complex diseases have a much smaller impact [61–63]. Clarify the role played by NOD2 polymorphisms in development of CRC still need further research [64].
In conclusion, regardless of age, gender, or presence of symptoms of subjects, deriving data from eight published articles, our meta-analysis suggests that R702W, G908R, and 3020insC increase the susceptibility to CRC in Caucasians, and P268S was not shown to impact on CRC risk. However, due to the small number of studies addressing this association of NOD2 and CRC, whether R702W, G908R, and 3020insC increase the susceptibility to CRC in other ethnicity and other NOD2 polymorphisms provide the same effects to CRC risk in different populations requires further investigation.
References
Capurso G, Marignani M, Delle Fave G (2006) Probiotics and the incidence of colorectal cancer: when evidence is not evident. Dig Liver Dis 38(Suppl 2):S277–S282
Kim HJ, Yu MH, Kim H, Byun J, Lee C (2008) Noninvasive molecular biomarkers for the detection of colorectal cancer. BMB Rep 41:685–692
Loffler I, Grun M, Bohmer FD, Rubio I (2008) Role of cAMP in the promotion of colorectal cancer cell growth by prostaglandin E2. BMC Cancer 8:380
Takahashi H, Inamori M (2009) Lifestyle-related disease and colorectal cancer. Intern Med 48:121
Kasztelan-Szczerbinska B, Cichoz-Lach H, Slomka M (2008) Colorectal cancer as a health care problem: evaluation of the current diagnostic options. Pol Arch Med Wewn 118:224–227
Velculescu VE (2008) Defining the blueprint of the cancer genome. Carcinogenesis 29:1087–1091
Hisamuddin IM, Yang VW (2004) Genetics of colorectal cancer. MedGenMed 6:13
Cheah PY (2009) Recent advances in colorectal cancer genetics and diagnostics. Crit Rev Oncol Hematol 69:45–55
Chu E (2009) Clinical colorectal cancer: "2008—the year in review". Clin Colorectal Cancer 8:9–10
Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S et al (2001) Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357:1925–1928
Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J et al (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599–603
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R et al (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606
Inohara C, McDonald C, Nunez G (2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355–383
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G et al (2003) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872
Inohara N, Nunez G (2001) The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 20:6473–6481
Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J et al (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278:5509–5512
Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR et al (2001) CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2:736–742
Angeletti S, Galluzzo S, Santini D, Ruzzo A, Vincenzi B, Ferraro E et al (2009) NOD2/CARD15 polymorphisms impair innate immunity and increase susceptibility to gastric cancer in an Italian population. Hum Immunol 70(9):729–732
Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD (1997) The codependence of angiogenesis and chronic inflammation. FASEB J 11:457–465
Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ (2000) Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60:184–190
Nakajima N, Kuwayama H, Ito Y, Iwasaki A, Arakawa Y (1997) Helicobacter pylori, neutrophils, interleukins, and gastric epithelial proliferation. J Clin Gastroenterol 25(Suppl 1):S198–S202
Bernstein CN, Blanchard JF, Kliewer E, Wajda A (2001) Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 91:854–862
Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI (2005) Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 100:2724–2729
Leshinsky-Silver E, Karban A, Buzhakor E, Fridlander M, Yakir B, Eliakim R et al (2005) Is age of onset of Crohn's disease governed by mutations in NOD2/caspase recruitment domains 15 and Toll-like receptor 4? Evaluation of a pediatric cohort. Pediatr Res 58:499–504
Alhopuro P, Ahvenainen T, Mecklin JP, Juhola M, Jarvinen HJ, Karhu A et al (2004) NOD2 3020insC alone is not sufficient for colorectal cancer predisposition. Cancer Res 64:7245–7247
Kurzawski G, Suchy J, Kladny J, Grabowska E, Mierzejewski M, Jakubowska A et al (2004) The NOD2 3020insC mutation and the risk of colorectal cancer. Cancer Res 64:1604–1606
Lakatos PL, Hitre E, Szalay F, Zinober K, Fuszek P, Lakatos L et al (2007) Common NOD2/CARD15 variants are not associated with susceptibility or the clinicopathologic characteristics of sporadic colorectal cancer in Hungarian patients. BMC Cancer 7:54
Papaconstantinou I, Theodoropoulos G, Gazouli M, Panoussopoulos D, Mantzaris GJ, Felekouras E et al (2005) Association between mutations in the CARD15/NOD2 gene and colorectal cancer in a Greek population. Int J Cancer 114:433–435
Roberts RL, Gearry RB, Allington MD, Morrin HR, Robinson BA, Frizelle FA (2006) Caspase recruitment domain-containing protein 15 mutations in patients with colorectal cancer. Cancer Res 66:2532–2535
Suchy J, Klujszo-Grabowska E, Kladny J, Cybulski C, Wokolorczyk D, Szymanska-Pasternak J et al (2008) Inflammatory response gene polymorphisms and their relationship with colorectal cancer risk. BMC Cancer 8:112
Szeliga J, Sondka Z, Jackowski M, Jarkiewicz-Tretyn J, Tretyn A, Malenczyk M (2008) NOD2/CARD15 polymorphism in patients with rectal cancer. Med Sci Monit 14:CR480–CR484
Tuupanen S, Alhopuro P, Mecklin JP, Jarvinen H, Aaltonen LA (2007) No evidence for association of NOD2 R702W and G908R with colorectal cancer. Int J Cancer 121:76–79
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 354:1896–1900
Hampe J, Grebe J, Nikolaus S, Solberg C, Croucher PJ, Mascheretti S et al (2002) Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet 359:1661–1665
Inohara N, Ogura Y, Chen FF, Muto A, Nunez G (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 276:2551–2554
Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276:4812–4818
Chen F, Castranova V, Shi X (2001) New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 159:387–397
Dejardin E, Deregowski V, Chapelier M, Jacobs N, Gielen J, Merville MP et al (1999) Regulation of NF-kappaB activity by I kappaB-related proteins in adenocarcinoma cells. Oncogene 18:2567–2577
Gilmore TD, Koedood M, Piffat KA, White DW (1996) Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene 13:1367–1378
Mukhopadhyay T, Roth JA, Maxwell SA (1995) Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene 11:999–1003
Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM et al (1997) Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest 100:2952–2960
Huzarski T, Lener M, Domagala W, Gronwald J, Byrski T, Kurzawski G et al (2005) The 3020insC allele of NOD2 predisposes to early onset breast cancer. Breast Cancer Res Treat 89:91–93
Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT et al (2006) Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol 7:27–38
Yang SK, Loftus EV Jr, Sandborn WJ (2001) Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis 7:260–270
Inoue N, Tamura K, Kinouchi Y, Fukuda Y, Takahashi S, Ogura Y et al (2002) Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123:86–91
Cavanaugh JA, Adams KE, Quak EJ, Bryce ME, O'Callaghan NJ, Rodgers HJ et al (2003) CARD15/NOD2 risk alleles in the development of Crohn's disease in the Australian population. Ann Hum Genet 67:35–41
Andersen V, Agerstjerne L, Jensen D, Ostergaard M, Saebo M, Hamfjord J et al (2009) The multidrug resistance 1 (MDR1) gene polymorphism G-rs3789243-A is not associated with disease susceptibility in Norwegian patients with colorectal adenoma and colorectal cancer; a case control study. BMC Med Genet 10:18
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J et al (1994) A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst 86:183–191
Ernst A, Jacobsen B, Ostergaard M, Okkels H, Andersen V, Dagiliene E et al (2007) Mutations in CARD15 and smoking confer susceptibility to Crohn's disease in the Danish population. Scand J Gastroenterol 42:1445–1451
Hansen RD, Sorensen M, Tjonneland A, Overvad K, Wallin H, Raaschou-Nielsen O et al (2008) A haplotype of polymorphisms in ASE-1, RAI and ERCC1 and the effects of tobacco smoking and alcohol consumption on risk of colorectal cancer: a Danish prospective case-cohort study. BMC Cancer 8:54
Anderson JC, Attam R, Alpern Z, Messina CR, Hubbard P, Grimson R et al (2003) Prevalence of colorectal neoplasia in smokers. Am J Gastroenterol 98:2777–2783
Liang PS, Chen TY, Giovannucci E (2009) Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 124:2406–2415
Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M et al (2000) Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343:78–85
Aaltonen L, Johns L, Jarvinen H, Mecklin JP, Houlston R (2007) Explaining the familial colorectal cancer risk associated with mismatch repair (MMR)-deficient and MMR-stable tumors. Clin Cancer Res 13:356–361
Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S et al (2008) Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet 40:1426–1435
Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, Spain S et al (2007) A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet 39:984–988
Zanke BW, Greenwood CM, Rangrej J, Kustra R, Tenesa A, Farrington SM et al (2007) Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet 39:989–994
Tomlinson IP, Webb E, Carvajal-Carmona L, Broderick P, Howarth K, Pittman AM et al (2008) A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet 40:623–630
Tenesa A, Farrington SM, Prendergast JG, Porteous ME, Walker M, Haq N et al (2008) Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet 40:631–637
Jaeger E, Webb E, Howarth K, Carvajal-Carmona L, Rowan A, Broderick P et al (2008) Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat Genet 40:26–28
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K (2002) A comprehensive review of genetic association studies. Genet Med 4:45–61
Ioannidis JP (2003) Genetic associations: false or true? Trends Mol Med 9:135–138
Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001) Replication validity of genetic association studies. Nat Genet 29:306–309
Guo QS, Xia B, Jiang Y, Qu Y, Li J (2004) NOD2 3020insC frameshift mutation is not associated with inflammatory bowel disease in Chinese patients of Han nationality. World J Gastroenterol 10:1069–1071
Acknowledgements
We thank Dr. Guan (Medical School, Nanjing University) for his critical review of the manuscript and evaluation of the statistical quality of the meta-analysis.
Declaration of personal interests
We are indebted to the authors of the primary studies
Declaration of funding interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, Y., Li, Y., Hu, Z. et al. Differential effects of NOD2 polymorphisms on colorectal cancer risk: a meta-analysis. Int J Colorectal Dis 25, 161–168 (2010). https://doi.org/10.1007/s00384-009-0809-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0809-9