Abstract
Objective
Lactic acid bacteria (LAB) can improve disturbances of indigenous microflora as well as inflammatory bowel diseases (IBD) such as ulcerative colitis and Crohn’s disease. We examined the anticolitic effect of Lactobacillus suntoryeus HY7801, which inhibited toll-like receptor (TLR)-4-linked NF-κB activation in human embryonic kidney (HEK) cells, in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitic mice.
Materials and methods
We measured the ability of commercial and intestinal LAB to inhibit lipopolysaccharide (LPS)-stimulated, TLR-4-linked NF-κB activation in HEK cells, as well as to inhibit colitis outcomes in TNBS-induced colitic mice. We also measured levels of the inflammatory markers, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, and their transcription factor, NF-κB, in intestinal mucosa by enzyme-linked immunosorbent assay and immunoblotting.
Results and discussion
LAB inhibited TLR-4-linked NF-κB activation, and L. suntoryeus HY7801 was the most potent inhibitor. Intrarectal treatment of TNBS in mice caused colon shortening and also increased colonic expression of IL-1β, IL-6, and TNF-α expression. However, oral administration of Lactobacillus HY7801 (100 mg/kg) inhibited colon shortening (p < 0.001) and myeloperoxidase activity in TNBS-induced colitic mice (p < 0.0002) and also decreased colonic expression of \(IL - 1\mathop \beta \limits_ \cdot \) (p < 0.003), IL-6 (p < 0.0001), and TNF-α (p < 0.0001). Lactobacillus HY7801 inhibited the NF-κB activation and TLR-4 expression induced by TNBS, as well as the expression of cyclooxygenase 2. Lactobacillus HY7801 also reduced the activity of intestinal bacterial glycosaminoglycan degradation and β-glucuronidase induced by TNBS.
Conclusion
L. suntoryeus HY7801 can improve colitis via the inhibition of TLR-4-linked NF-κB activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathogenic mechanism of inflammatory bowel diseases (IBD), such as ulcerative colitis and Crohn’s disease, involves the dysregulation of the intestinal immune response to intestinal environmental antigens, such as intestinal microflora [1–3]. IBD occurs most frequently in the terminal ileum and colon where many intestinal microbes reside [4, 5]. IBD does not significantly develop or progress in germ-free animals, indicating that intestinal microflora may play an important role in initiating and perpetuating colonic inflammation.
Normal intestinal microflora comprise an estimated 400 different bacterial species and reach their highest concentrations in the terminal ileum and colon [6, 7]. Intestinal microflora produce toxic compounds, such as gram-negative bacterial endotoxin, and harmful enzymes, such as β-glucuronidase and tryptophanase, which produce cytotoxic or carcinogenic agents [8–10]. Cytotoxins and endotoxins may interact at the apical intestinal surface and induce responses in intestinal epithelial cells, which produce pro-inflammatory cytokines and other mediators that induce inflammatory activation of the mucosal immune system via signaling through toll-like receptors (TLRs) and/or cytokine receptors [11]. TLRs, which serve as a major link between innate and adaptive mucosal immune responses, act as transmembrane coreceptors with CD14 in the cellular response to lipopolysaccharide (LPS) [12, 13]. TLR-4 is the primary mediator of LPS signaling [13, 14].
Lactic acid bacteria (LAB) are safe microorganisms [15] that improve disturbances of the indigenous microflora [16, 17], ameliorate the development of microflora [15], have antidiabetic and antihyperlipidemic effects [18, 19], inhibit carcinogenesis [17], have anticolitic effects [16, 20–22], and induce nonspecific activation of the host’s immune system [17]. Lactobacillus casei inhibits the expression of inflammatory cytokines in dextran sulfate sodium (DSS)-induced colitic mice [23]. Escherichia coli Nissle 1917, which activates TLR-4-linked NF-κB in human embryonic kidney (HEK) cells, ameliorates DSS-induced mouse colitis via TLR-2- and TLR-4-dependent pathways [24]. L. casei, Lactobacillus acidophilus, and Bifidobacterium lactis show intestinal anti-inflammatory activity in the TNBS model of rat colitis [25]. Nevertheless, the anticolitic mechanism of LAB has not been thoroughly examined.
Therefore, we tested whether LAB could inhibit TLR-4-linked NF-κB activation in HEK cells and examined their anticolitic effects in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitic mice.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), tetramethylbenzidine, Griess reagent, TNBS, hexadecyl trimethyl ammonium bromide, p-nitrophenyl-β-d-glucuronide, tryptophan, chondroitin sulfate, hyaluronic acid, and radio-immunoprecipitation assay (RIPA) lysis buffer were purchased from Sigma (St Louis, MO, USA). The protease inhibitor cocktail was purchased from Roche Applied Science (Mannheim, Germany). Enzyme-linked immunosorbent assay (ELISA) kits were from Pierce Biotechnology (Rockford, IL, USA). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The enhanced chemiluminescence (ECL) immunoblot system was from Pierce (Rockford, IL, USA).
The NF-κB reporter luciferase plasmid (pNiFty-luc) and a HEK293 cell line stably transfected with the TLR-4 gene (293-hTLR4A-HA cells) were purchased from InvivoGen (San Diego, CA, USA).
Bacterial strains and growth conditions
Four lactic acid bacterial strains were used in this study: LAB, Lactobacillus suntoryeus HY7801, Bifidobacterium longum HY130504, Lactobacillus plantarum AK8-3, and L. acidophilus A101, isolated by Korea Yakult, were grown anaerobically at 37°C in tryptic soy broth without shaking.
To prepare soluble fractions, cultured cells (6 × 109) were collected by centrifugation (10,000×g for 30 min) and washed twice with saline. The resulting pellet was suspended in phosphate-buffered saline (PBS). The cell suspension (5 mL) was placed in a 50-mL centrifuge tube, heated in boiling water for 10 min, and then centrifuged at 10,000×g for 60 min. The supernatant fraction of LAB was used for the experiments.
The cultured bacterial strains were grown to an optical density between 3 and 4 at 600 nm (early stationary phase), harvested by centrifugation (10,000×g for 30 min), washed with PBS, and then orally administered to mice as a suspension in 0.2 M NaHCO3 buffer containing 1% glucose [20, 26].
Transient transfection of NF-κB luciferase reporter into 293-hTLR4-HA cells and luciferase activity assay
293-hTLR4A-HA cells (InvivoGen) in 10-cm plates (1 × 105 cells/mL) were transiently transfected with the pNiFty-luc (InvivoGen) luciferase reporter plasmid (24 μg/plate) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 6 h, the media was changed and the cells were transferred to 96-well plates. After 18 h, the cells were stimulated with LPS (1 μg/mL) and heat-inactivated LAB (1 × 105/mL) for 6 h. The cells were subsequently harvested and lysed, and luciferase activity was measured (Synergy HT, Bio-Tek) using the Bright-Glo™ Luciferase Assay System purchased from Promega (Madison, WI, USA).
Animals
Male ICR mice (24–28 g) were supplied from Jung-Ang Lab Animal (Seoul, South Korea). All animals were housed in wire cages at 20–22°C and 50 ± 10% humidity, fed standard laboratory chow (Samyang, Seoul, South Korea), and allowed water ad libitum. All procedures relating to the animals and their care conformed to the international guidelines, “Principles of Laboratory Animals Care” (NIH publication no. 85-23 revised 1985 and Kyung Hee University 2006).
Preparation of experimental colitic mice
Male ICR mice were randomly divided into five groups: normal and TNBS-induced colitic groups treated with or without LAB or sulfasalazine. TNBS-induced colitis was induced by the administration of 2.5% (w/v) TNBS solution (100 μL) in 50% ethanol into the colon of lightly anesthetized mice via a thin round-tip needle equipped with a 1-mL syringe [27]. The normal group was treated with vehicle alone. The needle was inserted so that the tip was 3.5–4 cm proximal to the anal verge. To distribute the agents within the entire colon and cecum, mice were held in a vertical position for 30 s after the injection. Using this procedure, >95% of the mice retained the TNBS enema. If an animal quickly excreted the TNBS–ethanol solution, it was excluded from the remainder of the study. Lactobacillus HY7801 [2 × 1010 (50 mg) or 4 × 1010 (100 mg) colony forming unit (CFU) per kilogram] were orally administered once a day from 3 days before TNBS treatment to the day before killing the mice. The mice were anesthetized with ether and killed on the third day after TNBS administration. The colon was quickly removed, opened longitudinally, and gently cleared of stool by PBS. Macroscopic assessment of the disease grade was scored according to a previously reported scoring system (0, no ulcer and no inflammation; 1, ulceration and local hyperemia; 2, ulceration without hyperemia; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation; 5, ulceration extending more than 2 cm [28]), and the colon tissue was then used for immunoblot and ELISA analysis.
Assay of myeloperoxidase activity in colonic mucosa
Colons were homogenized in a solution containing 0.5% hexadecyl trimethyl ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7.0), and then centrifuged for 30 min at 20,000×g and 4°C. An aliquot (50 μL) of the supernatant was added to a reaction mixture of 1.6 mM tetramethylbenzidine and 0.1 mM H2O2 and incubated at 37°C; the absorbance was obtained at 650 nm over time. Myeloperoxidase (MPO) activity was defined as the quantity of enzyme degrading 1 μmol/mL of peroxide at 37°C and expressed in unit per milligram protein [29]. The protein content was assayed by the Bradford method [30].
ELISA and immunoblot
For the ELISA of IL-1β, TNF-α and IL-6, colons were homogenized in 1 mL of ice-cold RIPA lysis buffer containing 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. The lysate was centrifuged (15,000×g, 4°C) for 15 min, and the supernatant was transferred to 96-well ELISA plates. IL-1β, TNF-α and IL-6 concentrations were determined using commercial ELISA kits (Pierce Biotechnology, Rockford, IL, USA).
For immunoblotting of TLR-4, pp65 (phospho-NF-κB), p65 (NF-κB), cyclooxygenase (COX)-2 and β-actin, the colon tissue was carefully homogenized to obtain viable single cells, which were resuspended in 1 mL of RIPA lysis buffer containing 1% a protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. After centrifugation, the supernatant was used for the immunoblot assay. The protein from collected cells was subjected to electrophoresis on an 8–10% sodium dodecyl sulfate polyacrylamide gel and then transferred to nitrocellulose membrane. Levels of pp65, p65, COX-2, and β-actin were assayed as previously described [31]. Immunodetection was performed using an enhanced chemiluminescence detection kit.
Preparation of fecal bacterial suspension
Fresh mouse stools (0.5 g) from each group were separately collected in sterilized plastic cups, carefully suspended with 20-fold saline in a cooled tube, and centrifuged at 250×g for 5 min. The supernatant was recentrifuged at 10,000×g for 20 min. The resulting precipitates were used as the sources for the fecal enzyme assays. All procedures were performed at 4°C.
β-Glucuronidase activity assay
The reaction mixture (1.0 mL), consisting of 0.04 mL of 2 mM p-nitrophenyl-β-d-glucuronide, 0.76 mL of 0.1 M phosphate buffer (pH 7.0), and 0.2 mL of fecal suspension, was incubated for 30 min at 37°C, and the reaction was terminated by the addition of 1 mL of 0.5 M NaOH. The mixture was then centrifuged at 3,000×g for 10 min and the absorbance was measured at 405 nm.
Chondroitin sulfate and hyaluronic acid degradation assays
Reaction mixtures containing 0.2 mL chondroitin sulfate A (or hyaluronic acid; 1.0 mg/mL) and 0.6 mL of the fecal suspension were incubated at 37°C for 1 h and then centrifuged at 3,000×g at 4°C. The supernatant (500 μL), 0.1 mL of 0.4 M NaOH, and 0.1 mL of 0.4 M potassium borate were boiled for 5 min and cooled to room temperature; 3 mL of 67 mM p-dimethylaminobenzaldehyde was then added. The mixture was incubated at 37°C for 20 min, and the absorbance was measured at 585 nm.
Statistical analysis
All data are expressed as the mean±standard deviation with statistical significance analyzed using one-way ANOVA followed by a Student–Newman–Keuls test.
Results
LAB inhibits TLR-4-linked NF-κB activation in 293-hTLR4-HA cells
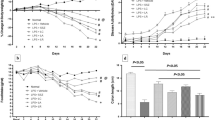
We evaluated the ability of commercial and intestinal LAB to inhibit TLR-4-linked NF-κB activation using HEK293 cells transfected with an NF-κB luciferase reporter construct (293-hTLR4-HA cells) (Fig. 1). LPS treatment significantly induced NF-κB luciferase reporter activity, and LAB inhibited this activity. Lactobacillus HY7801 (1 × 105/mL) inhibited this activity most potently by 65% (p < 0.001). LAB treatment alone slightly increased NF-κB activation.
Commercial and intestinal LAB inhibit LPS-stimulated, TLR4-linked NF-κB activation in HEK cells. Cultured LAB were collected by centrifugation (10,000×g for 30 min) and washed twice with PBS. The resulting pellet was suspended in PBS. The cell suspension was placed into a 50-mL centrifuge tube, heated in boiling water for 10 min, and then centrifuged at 10,000×g for 30 min. The resulting pellets (1 × 105/mL) were suspended in PBS and used as the test agents. N normal control, L treated with LPS alone, L+L7 treated with L. suntoryeus HY7801 and LPS, L+B5 treated with B. longum HY 130504 and LPS, L+Lp treated with L. plantarum AK8-3 and LPS, L+La treated with L. acidophilus A101 and LPS. Enzyme activity values are the mean±SD (n = 3). Items with different letters for colon length are significantly different (p < 0.05)
Anti-inflammatory effect of Lactobacillus HY7801 in experimental colitic mice
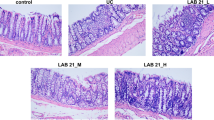
We next tested the ability of Lactobacillus HY7801, which potently inhibited NF-κB activation, to inhibit colitic activity induced by TNBS. TNBS induced loss of body weight and severe inflammation, manifested by shortened, thickened, and erythematous colons from the first day following oral administration of TNBS (Fig. 2). Colon histology showed massive bowel edema, dense infiltration of the superficial layers of the mucosa, and epithelial cell disruption by large ulcerations. Lactobacillus HY7801 treatment inhibited body weight reduction (100 mg/kg, (p < 0.0005), colon shortening (100 mg/kg, p < 0.001), inflammation, and thickening on the third day after TNBS treatment. Lactobacillus HY7801 treatment (100 mg/kg) also inhibited TNBS-induced MPO activity, an inflammatory marker, by 86% in colon epithelial cells (p < 0.0002). Lactobacillus HY7801 showed greater potency than sulfasalazine, a commercial medicine.
The effects of LAB on colon length (a), macroscopic disease (b), colonic MPO activity (c), and body weight (d) in TNBS-induced colitic mice. TNBS, except in the normal group (N normal group treated with vehicle alone), was intrarectally administered in control (C), LAB, and sulfasalazine groups. LAB (LL 50 mg/kg L. suntoryeus HY7801 treated with TNBS, LH 100 mg/kg of Lactobacillus HY7801 treated with TNBS) or sulfasalazine (S, 50 mg/kg) treated with TNBS, except in the normal and control groups, was orally administered from 3 days prior to TNBS treatment. The mice were anesthetized with ether and killed 3 days after TNBS treatment. All values are the mean±SD (n = 10). #p < 0.05, significantly different vs. normal group; *p < 0.05, significantly different vs. control group
We next measured the levels of the pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6, in the colons of TNBS-induced colitic mice (Fig. 3). TNBS increased protein expression of IL-1β, IL-6, and TNF-α by 3.1-fold, 15.7-fold, and 3.0-fold, respectively. Lactobacillus HY7801 treatment inhibited these cytokine expressions, but did not affect β-actin expression. Treatment with Lactobacillus HY7801 (100 mg/kg) inhibited these cytokine expressions by 59% (p < 0.003), 94% (p < 0.0001), and 95% (p < 0.0001), respectively.
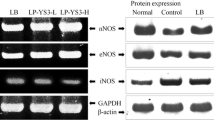
The effect of LAB on TLR-4 and COX-2 expressions and NF-κB activation (a) and inflammatory cytokines (b) in TNBS-induced colitic mice. The test agents (N normal vehicle alone, C TNBS-treated control group, LL 50 mg/kg L. suntoryeus HY7801 treated with TNBS, LH 100 mg/kg of Lactobacillus HY7801 treated with TNBS, S 50 mg/kg sulfasalazine treated with TNBS) were orally administered from 3 days prior to TNBS treatment. The mice were anesthetized and killed 3 days after TNBS treatment. Colon epithelial cells were collected and the TLR-4 and COX-2 expression and the NF-κB activation were measured by immunoblot analysis, and IL-1β and IL-6 were measured by ELISA. All values are the mean±SD (n = 10). #p < 0.05, significantly different vs. normal group; *p < 0.05, significantly different vs. control group
TNBS also increased the expression of TLR-4, an LPS receptor in the NF-κB signaling pathway [12], as well as of NF-κB (pp65) and COX-2. Lactobacillus HY7801 blocked the induction of TLR-4, pp65, and COX-2 TNBS. Lactobacillus HY7801 was more potent than sulfasalazine.
Lactobacillus HY7801 inhibits harmful enzyme activities of intestinal microflora in experimental colitic mice
TNBS induced β-glucuronidase activity, as well as glycosaminoglycan (GAG; chondroitin sulfate and hyaluronic acid) degradation (Fig. 4). Lactobacillus HY7801 significantly reduced these enzyme activities with greater potency than sulfasalazine, a commercial medicine. Of these enzyme activities, Lactobacillus HY7801 most potently inhibited hyaluronic acid degradation (p < 0.04) with treatment at a dose of 100 mg/kg reducing the activity to that of the normal group.
The effects of LAB on fecal β-glucuronidase (a), hyaluronidase degradation (b), and chondroitin degradation (c) activities in TNBS-induced colitic mice. Test agents (N normal group treated vehicle alone, C TNBS-treated control group, LL 50 mg/kg L. suntoryeus HY7801 treated with TNBS, LH 100 mg/kg of Lactobacillus HY7801 treated with TNBS, S 50 mg/kg sulfasalazine treated with TNBS) were orally administered from 3 days prior to TNBS treatment. Enzyme activities are the mean±SD (n = 10). #p < 0.05, significantly different vs. normal group; *p < 0.05, significantly different vs. control group
Discussion
Inflammatory bowel disease is a severe form of intestinal inflammation with unclear pathogenesis resulting in part from complex mucosal immune responses to resident enteric bacteria [11, 32]. The innate immune system recognizes the presence of specific bacterial antigens through an extensive family of pattern recognition receptors [12–14]. TLR-4 is a pattern recognition receptor that responds to LPS, a constituent of gram-negative bacteria that activates the secretion of pro-inflammatory mediators from monocytes and dendritic cells, leading to the activation of the acquired immune response. TLR-4 signaling may help maintain an ongoing inflammatory response. Indeed, TLR-4 overexpression is observed in intestinal epithelial cells from the colons of patients with IBD [14]. TLR-4 is also upregulated during DSS-induced colitis in mice and NF-κB, which is a representative transcription factor regulating the expression of pro-inflammatory cytokines, is also activated [14]. Similarly, we also observed that TNBS not only upregulated the TLR-4 expression in the colon, but also activated the transcription factor, NF-κB. TNBS also induced COX-2 expression, which is regulated via TLR-4-linked NF-κB activation. These results suggest that TNBS may upregulate TLR-4 expression and that intestinal bacterial LPS may deteriorate TNBS-induced colitis via TLR-4-linked NF-κB activation. Pro-inflammatory cytokines may be upregulated via NF-κB activation by TNBS.
The probiotics, bifidobacteria and lactobacilli, suppress the growth of pathogens by releasing antimicrobial factors that compete with microbial pathogens for the limited number of receptors on epithelial cells [33, 34]. Several studies have clearly demonstrated the beneficial effects of probiotics in the treatment of IBD [15, 16]. Probiotics may be an alternative, safe treatment modality for IBD. VSL#3 increases the expression of IL-10, an anti-inflammatory cytokine [35]. L. casei inhibits the expression of inflammatory cytokines in DSS-induced colitic mice [23]. E. coli Nissle 1917 improves DSS-induced colitis via TLR-2- and TLR-4-dependent pathways, although it activates TLR-4-linked NF-κB [13].
We found that many LAB inhibited TLR-4-linked NF-κB activation in a cell system. Of the LAB tested, Lactobacillus HY7801 most potently inhibited TLR-4-linked NF-κB activation and also blocked TNBS-induced colitis symptoms, including diarrhea and intestinal shortening. In addition, Lactobacillus HY7801 blocked TNBS-stimulated MPO activity, an index of polymorphonuclear leukocyte accumulation, in the intestines, as well as the expression of IL-1β, IL-6, and TNF-α, as previously reported [36, 37]. These results suggest that Lactobacillus HY7801 may inhibit TNBS-induced colitis by regulating NF-κB activation and TLR-4 expression. Lactobacillus HY7801 also inhibited harmful intestinal bacterial enzyme activities, such as β-glucuronidase, tryptophanase, and GAG degradation, which produce carcinogenic and cytotoxic metabolites. These results suggest that Lactobacillus HY7801 may inhibit the growth of harmful intestinal bacteria and/or their production of harmful enzymes.
Based on these findings, Lactobacillus HY7801 may be able to improve colitis via inhibition of TLR-4-linked NF-κB activation and harmful enzyme production of intestinal bacteria.
Abbreviations
- IBD:
-
inflammatory bowel disease
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ECL:
-
enhanced chemiluminescence
- ELISA:
-
enzyme-linked immunosorbent assay
- HEK:
-
human embryonic kidney
- IL:
-
interleukin
- LAB:
-
lactic acid bacteria
- LPS:
-
lipopolysaccharide
- RIPA:
-
radio-immunoprecipitation assay
- TLR:
-
toll-like receptor
- TNBS:
-
2,4,6-trinitrobenzenesulfonic acid
- TNF:
-
tumor necrosis factor
References
Benno P, Leijonmarck CE, Monsen U, Uribe A (1993) Functional alteration of the microflora in patients with ulcerative colitis. Scand J Gastroenterol 28:839–844
Berrebi D, Languepin J, Ferkdadji L, Foussat A, De Lagausie P, Paris R, Emilie D, Mougenot JF, Cezard JP, Navarro J, Peuchmaur M (2003) Cytokines, chemokine receptors, and homing molecule distribution in the rectum and stomach of pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr 37:300–308
Gorbach SL, Nahas L (1968) Studies of intestinal microflora. V. Fecal microbial ecology in ulcerative colitis and regional enteritis, relationship to severity of disease and chemotherapy. Gastroenterology 54:575–587
Binder V (2004) Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol 18:463–479
Chandran P, Satthaporn Robins A, Eremin O (2003) Inflammatory bowel disease: dysfunction of GALT and gut bacterial flora (II). Surgeon 1:125–136
Hill MJ, Drasar BS (1975) The normal colonic bacterial flora. Gut 16:318–323
Simon GL, Gorbach SL (1984) Intestinal flora in health and disease. Gastroenterology 86:174–193
Chung KT, Fulk GE, Slein MW (1975) Tryptophanase of fecal flora as a possible factor in the etiology of colon cancer. J Natl Cancer Inst 54:1073–1078
Ganguly NK, Kingham JG, Lloyd B, Lloyd RS, Price CP, Triger DR, Wright R (1978) Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet 1(8073):1073–1075
Rhodes JM, Gallimore R, Elias E (1985) Fecal mucus degrading glycosidase in ulcerative colitis and Crohn’s disease. Gut 26:761–765
Jung HC, Eckmann I, Yang SK, Panja A, Fierer J, Morzycka-Worblewska E, Kagnoff MF (1995) A distinct array of proinflammatory cytokine is expressed in human colon epithelia cells in response to bacterial invasion. J Clin Invest 95:55–65
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F (1999) Toll-like receptor-4 mediates lipolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692
Ingalls RR, Heine H, Lien E, Yoshimura A, Glenbock D (1999) Lipopolysaccharide recognition, CD14, and lipopolysaccharide receptors. Infect Dis Clin North Am 13:341–353
Cario E, Pldolsky DK (2000) Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68:7010–1017
Collins MP, Gibson GR (1999) Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69:s1052–s1057
Campieri M, Gionchetti P (1999) Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative. Gastroenterology 116:1246–1260
Perdigon G, de Jorrat WEB, de Petrino SF, Valerde de Budeguer M (1991) Effect of oral administration of Lactobacillus casei on various biological functions of the host. Food Agric Immunol 3:93–102
Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, Hosono A (2003) Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 67:1421–1424
Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF (1998) Evidence for hypoglycemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci 81:2336–2340
Daniel C, Poiret S, Goudercourt D, Dennin V, Leyer G, Pot B (2006) Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol 72:5799–5805
Han W, Mercenier A, Ait-Belgnaoui A, Pavan A, Lamine F, van Swam II, Kleerebezem M, Salvador-Cartier C, Hisbergues M, Bueno L, Theodorou V, Fioramonti J (2006) Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis 12:1044–1052
Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailon E, Nieto A, Olivares M, Zarzuelo A, Xaus J, Galvez J (2007) A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr 97:96–103
Chung YW, Choi JH, Oh TY, Eun CS, Han DS (2007) Lactobacillus casei prevents the development of dextran sulfate sodium-induced colitis in toll-like receptor 4 mutant mice. Clin Exp Immunol 151:182–189
Grabig A, Pcclik D, Guzy C, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, Sonnenborn U, Eckert J, Schumann RR, Wiedenmann B, Dignass AU, Sturm A (2006) Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun 74:4075–4082
Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J (2007) A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol 103:836–844
Han SY, Huh CS, Ahn YT, Lim KS, Baek YJ, Kim DH (2005) Hepatoprotective effect of lactic acid bacteria. J Microbiol Biotechnol 15:887–890
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT (2006) Cox-2 is regulated by toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131:862–877
Hollenbach E, Vieth M, Rosessner A, Neumann M, Malfertheiner P, Naumann M (2005) Inhibition of RICK/Nuclear factor-kB and p38 signalling attenuates the inflammatory response in a murine model of Crohn Disease. J Biol Chem 280:14981–14988
Mullane KM, Kraemer R, Smith B (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14:157–167
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Shin YW, Bae EA, Kim SS, Lee YC, Kim DH (2005) Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis. Int Immunopharmacol 5:1183–1191
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewk K, Meyer zum Buschenfelde KH (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol 102:448–455
Kruis W (2004) Antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 20(suppl. 4):75–78
Sartor RB (2004) Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics and prebiotics. Gastroenterology 126:1620–1633
Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM (2007) VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther 6:1449–1454
Bai A, Lu N, Guo Y, Fan X (2008) Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Dig Dis Sci 53:421–428
Barthet M, Dubucquoy L, Garcia S, Gasmi S, Descreumaux P, Colombel JF, Grimaud JC, Iovanna J, Dagorn JC (2003) Pancreatic changes in TNBS-induced colitis in mice. Gastroenterol Clin Biol 27:895–900
Acknowledgement
This study was supported by a grant from Korea Yakult (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JH., Lee, B., Lee, HS. et al. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. Int J Colorectal Dis 24, 231–237 (2009). https://doi.org/10.1007/s00384-008-0618-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0618-6