Abstract
Background and aims
Checkpoint kinases 1 and 2 (Chk1 and Chk2) are emerging as key mediators in diverse cellular responses to genotoxic stress, guarding the integrity of the genome. Recent studies suggest the fundamental role of Chk1 and Chk2 in the network of genome surveillance pathways which coordinate cell cycle progression with DNA repair and cell survival or death. Defects in these two serine/threonine kinases are suggested contributors to the development of both hereditary and sporadic human cancer. Little is known about physiologic activities of Chk1 and Chk2 in the colorectal cancer or their role in tumorigenesis.
Patient/methods
Expression of Chk1 and Chk2 and their phosphorylated, i.e., active forms (pChk1, pChk2) was examined by Western blot and ELISA analysis in colorectal carcinomas and normal colonic mucosa.
Results/findings
Expression of Chk2 and pChk2 was noted to be decreased in around 50% of studied cancer cases. Quantitative studies of phosphorylated Chk2 revealed significant decrease of pChk2 in early stages of colorectal carcinomas. Furthermore, tumor invasion to local lymph nodes was found to correlate with the increase of pChk2 pool in the studied cases.
Interpretation/conclusion
Reduced expression of Chk2 and activated Chk2 may be an important inactivating mechanism, contributing to the development of colorectal neoplasm. However, during progression of neoplasia, activated Chk2 may contribute to the invasiveness of tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental factors including ultraviolet (UV) light, ionizing radiation, and oxidative stress continually damage the genome of cells. In dividing cells, it is also prone to the introduction of errors during DNA replication and mitosis. To ensure the stability of the genome, cells activate complex signaling networks in response to DNA damage and replication stress. These pathways, termed checkpoint signaling pathways, first detect the damage and then transduce the signal to downstream substrates, i.e., the ataxia telangiectasia mutated/ataxia telangiectasia and rad3 related (ATM/ATR) and checkpoint kinases 1/2 (Chk1/Chk2 kinases) which are central players in checkpoint activation in response to DNA damage [1–4].
The choice of damage signal transducers reflects the type of DNA damage, though some overlap between the ATM-Chk2 axis and the ATR-Chk1 axis exists [5, 6]. Chk1 is required for multiple DNA-damage checkpoints. Chk1 is phosphorylated by the orthologues of ATR and/or ATM at Ser317 in response to hydroxyurea (HU) and UV, and Ser345 in response to HU, UV, and ionizing radiation (IR) [7–10]. In addition to ATR, two protein complexes, Rad17/replication factor (RFC) and Rad9/hydroxyurea sensitive 1 (Hus1)/Rad1 (9-1-1), are required for Chk1 activation, but it is not clear how this collaboration in Chk1 activation is achieved [3, 9, 11]. Chk2 kinase is activated (phosphorylated) in response to IR in an ATM-dependent manner and in response to UV and treatment with replication inhibitors in an ATM-independent and probably ATR-dependent manner [12–14]. ATM phosphorylates Chk2 on Thr68 and thereby promotes the oligomerization of Chk2 by creating a specific binding site for the forkhead-associated (FHA) domain of another Chk2 molecule. Homodimerization of Chk2 also facilitates trans-phosphorylation of the C-terminal kinase domain (Thr383, Thr387), which is required for full activation of Chk2 [2, 4, 12]. Although Chk1 is structurally unrelated to Chk2 outside the kinase domain, both proteins can phosphorylate a similar set of substrates. Both kinases phosphorylate and inactivate Cdc25 (cell-division cycle) phosphatase. Chk1 and Chk2 proteins phosphorylate Cdc25A on multiple serine residues, which lead to enhanced ubiquitination and proteasome-mediated degradation of Cdc25A. Suppression of Cdc25A promotes inhibitory phosphorylation and inactivation of cyclin E/Cdk2 (cyclin-dependent kinase) and cyclin A/Cdk2 kinases required for entering into and progression through the S phase in mammalian cells [5, 6, 12, 15, 16]. At the same time, the phosphorylation of Cdc25C leads to inactivation of this phosphatase either directly or indirectly through the creation of a 14-3-3 protein binding site. This interaction results in persistent cytoplasmic Cdc25C localization thus preventing the G2/M transition as the cyclin B/Cdk1 complex cannot be activated. Another target proposed for Chk1 and Chk2 is the tumor suppressor p53, a transcription factor that functions in response to DNA damage occurring in G1/S phase. Phosphorylation of p53 (on Thr18, Ser20, and possibly other residues) by Chk1/Chk2 stabilizes p53 protein, which enhances its potential to positively regulate the expression of factors involved in cell cycle arrest, DNA repair and apoptosis. Stabilization of p53 also results from reduced association with Mdm2 (mouse double minute protein 2), which promotes the p53 degradation [1, 2, 5, 6, 12, 15, 17].
Chk1 is essential for embryonic development, which suggests that Chk1 function is not only required upon genotoxic assaults but also under normal physiological conditions. Chk1 was found to be phosphorylated in the absence of exogenous genotoxic agents, possibly due to signals generated by stalled replication forks occurring in each cell cycle [18, 19] (Fig. 1).
Chk2 acts as a “signal spreader” dispersing a checkpoint signal to downstream targets, such as p53, Cdc25A, Cdc25C, BRCA1 (breast-cancer susceptibility gene 1), and E2F-1 (cell-cycle-regulated transcription factor) [2, 5, 6, 12, 15]. Chk2 phosphorylates E2F-1 at Ser364 and this modification regulates E2F-1 stabilization and transcriptional activity [12, 20]. Overexpression of E2F-1 can induce apoptosis or prevent G1/S transition.
Defects in Chk1 and Chk2 kinases are suggested contributors to the development of both hereditary and sporadic human cancers [21–30]. In this report alternations in the expression of Chk1 and Chk2 and their phosphorylated forms in colorectal cancer and their potential significance for colorectal tumorigenesis were studied.
Materials and methods
Patients
The tissue specimens were supplied by the Department of General and Colorectal Surgery, Medical University in Lodz, Poland. Before the operation, all patients underwent preoperative assessment of cancer staging according to Dukes’, TNM, and UICC classification. Endoscopy, computed tomography, and rectal ultrasound were routinely performed. Thirty percent of patients were diagnosed with heart disease or hypertension, 20% have intestinal diverticular disease, and 40% suffered from constipation. Patients underwent surgery and tumors were resected with an adequate surgical margin and examined by pathologists postoperatively. Studied samples were identified as sporadical primary tumors. The patients did not receive chemo- or radiotherapy prior to surgical intervention. The specimens were frozen at −70°C immediately after the surgery. Thirty-eight fresh frozen samples: 17 colorectal carcinomas, two hyperplasic tissues, and 19 normal colonic mucosas were analyzed. The clinical characteristics of studied material are shown in Table 1.
Samples preparation
Briefly, the tissue was minced and homogenized with the use of a motor driven Potter homogenizer in 10 volumes of lysis buffer: 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Igepal, 40 nM bpV(phen), 0.1 μg/ml okadaic acid, 50 mM sodium fluoride, 2 nM sodium orthovanadate and 30 mM sodium pyrophospate, and 50 mM Tris-HCl at pH 8.0.
The efficiency of homogenization was monitored by phase-contrast microscopy. The homogenate was centrifuged at 800 g for 10 min. Concentration of total protein pool was evaluated in each sample using modified Lowry protocol [31].
Antibodies
Qualitative and quantitative analysis of Chk1 and Chk2 and their phoshorylated forms, i.e. pChk1 and pChk2, respectively was performed by Western blot and enzyme-linked immunosorbent assay (ELISA) techniques in the presence of the following commercially available antibodies: monoclonal anti-Chk1 antibody against amino acids 1-476 representing full length of human Chk1 protein; monoclonal anti-Chk2 antibody against amino acids 1-300 of human Chk2 protein; polyclonal anti-Chk1 (Ser345) antibody, against epitope corresponding to a short amino acid sequence surrounding phosphorylated Ser345 of human Chk1 protein (Santa Cruz Biotechnology Inc., USA); polyclonal anti-Chk2 (Thr68) antibody, against epitope corresponding to amino acid sequence surrounding phosphorylated Thr 68 residue of human Chk2 protein (Cell Signaling Technology Inc., USA); and polyclonal swine anti-goat, mouse, rabbit immunoglobulins/biotinylated (Dako, Denmark). A polyclonal antibody anti-actin (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) was used as a loading control.
Western blot
Separation of proteins (20 μg/well) was performed on 7.5% SDS-PAGE (20–40 mA/gel) in electrophoretic buffer (0.1% SDS, 192 mM glycine, 25 mM Tris, pH 8.3 [32]. Electrophoretically separated proteins were electroblotted using a semi-dry system to Immobilon P membranes (pore size, 0.45 μm; Millipore, USA) in transfer buffer containing 20% (v/v) methanol, 192 mM glycine, 25 mM Tris, pH 8.3 [33, 34]. The membranes were incubated for 1 h at room temperature in 0.5% non-fat dry milk or 1% gelatin in Tris-buffered saline with Tween 20 (TBST, 150 mM NaCl, 0.05% Tween 20, 100 mM Tris-HCl, pH 7.4) to saturate non-specific protein binding sites.
The membranes were incubated with specific primary antibodies diluted in TBST at 4oC for immunodetection of the studied proteins. The antigens stacked on the membrane were identified in the presence of StreptABComplex/horseradish peroxidase (HRP) (Dako, Denmark). 3,3’-diaminobenzidine (DAB) tablets and H2O2 (Sigma Fast™) were used as a substrates for the localization of HRP activity. Qualitative and quantitative analysis was performed by measuring integrated optical density (IOD) by GelProAnalyzer vel. 3.0 for Windows™ program (Media Cybernetics, USA).
ELISA assay
ELISA test was performed in the presence of primary antibodies, StreptABComplex/HRP detection system (Dako, Denmark), and o-phenylenediamine dihydrochloride (OPD; SigmaAldrich, Germany) as a substrate. Ninety-six-well plates were coated with an antigen solution at concentration of 10 μg/well in 50 mM carbonate buffer, pH 9.6. Absorbance was read in a microplate reader at 490 nm (Micro Plate Reader, BioRad, USA).
Optimal concentration for each examined protein and antibodies was established by titration. The same protocol was used for negative controls without antigen, specific antibody, biotinylated immunoglobulins, and StreptABC/HRP complex.
Statistical analysis
All data were presented as mean ± SEM. One-way ANOVA with Dunnett’s post-test and unpaired t-test were performed using GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com. A P value <0.05 was considered statistically significant.
Results
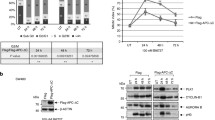
Expression of Chk1, Chk2, and their activated (phosphorylated) forms pChk1 and pChk2 was examined by Western blot (Fig. 2) and ELISA test. Expression of the studied proteins was observed in all tumor (n = 17) and corresponding normal mucosa (n = 19) samples. In 14% by Western blot and 21% by ELISA no changes in the amount of Chk1 and Chk2 were observed between colorectal cancer and normal mucosa (Table 2). However, Chk1 protein level increased in 50% by Western blot and 43% by ELISA of tumor samples as compared to normal mucosa (Table 2). Contrary activated form of Chk1 was found to decrease in 56% and 52% of tumor samples by Western blot and ELISA, respectively (Table 3). In 17% and 26% of tumor samples by Western blot and ELISA, respectively no changes in relative amount of pChk1 were observed. Analysis of Chk1 and pChk1 expression demonstrated no statistically significant differences in relation to normal mucosa as well as clinicopathological features of cancer (Fig. 3).
Qualitative analysis by Western blot of Chk1/pChk1 and Chk2/pChk2 in normal mucosa N and colorectal cancer samples staged according to the classification of Dukes (A, B, C). Actin served as control for the amount of protein loading. Antibodies are described in “Material and methods” section
Quantitative analysis by ELISA of Chk1 (A) and pChk1 (B) protein level in normal mucosa N (n = 19) and colorectal cancer T (n = 17) staged according to the classification of Dukes A (n = 7), B (n = 5), C (n = 5) and lymph node invasiveness status, i.e. negative N = 0 (n = 5) and positive i.e. N > 0 (n = 11)
In the case of Chk2 decreased protein level was noted in 50% by Western blot and 57% by ELISA of tumor samples as compared to normal tissue (Table 2). Similarly, decreased level of activated (phosphorylated) in comparison to not activated form of Chk2 was observed in 50% by Western blot and 42% by ELISA of tumor samples. In 22% and 26% by Western blot and ELISA respectively of studied cases no changes in protein level was found (Table 3). Quantitative analysis of studied protein revealed that the decrease of pChk2 in cancer specimens in relation to normal mucosa is statistically significant (Fig. 4). When pChk2 protein level was analyzed in respect of tumor clinical stage and node invasiveness, statistically significant decrease seems to be characteristic for early stages of tumorigenesis, i.e., stage A and B according to Dukes classification, and stage N0 (without lymph node metastasis) according to TNM classification. Moreover, significant increase in pChk2 protein level seems to accompany tumor invasion to lymph nodes (Fig. 4). In the case of two hyperplasic tissue samples, the pattern of activated and not activated forms of Chk1 and Chk2 reassembled the pattern of early stages of colorectal carcinomas.
Quantitative analysis by ELISA of Chk2 (A) and pChk2 (B) protein level in normal mucosa N (n = 19) and colorectal cancer T (n = 17) staged according to the classification of Dukes A (n = 7), B (n = 5), C (n = 5) and lymph node invasiveness status, i.e. negative N = 0 (n = 5) and positive i.e. N > 0 (n = 11) *P < 0.05
Discussion
The central role of the kinases Chk1 and Chk2 in DNA damage response pathways, downstream of ATM and upstream of p53, and the detection of Chk1 and Chk2 genes mutations in a subset of families affected by Li–Fraumeni syndrome have suggested that these genes may be a potential tumor suppressors involved in the pathogenesis of sporadic tumors.
To determine the potential role of Chk1 and Chk2 alterations in the pathogenesis of colorectal neoplasms we examined level of these proteins in a series of colorectal tumor samples. Several studies analyzed the mutational status of Chk1 and Chk2 in human neoplasms [21, 22, 25–29]. However, the information on protein expression in different types of cancer is very limited [23–25, 35, 36]. Mutation of Chk1 was not observed in microsatellite instability-positive colorectal, stomach, and endometrial cancers, indicating that mutational inactivation of Chk1 rarely occurs in cancers of the digestive tract [37]. Other studies confirmed low frequency of mutations within the Chk1 gene in sporadic stomach tumors with microsatellite instability (2/23) and in genetically unstable colorectal (1/10) and endometrial cancers (2/7) [21, 22]. If mutational inactivation of Chk1 is rare in digestive tract, the functional abilities of Chk1 to prevent cell cycle progression may be preserved [24]. To examine the function of Chk1 protein in the colorectal cancer we compared the expression patterns of Chk1 and phosphorylated Chk1 protein in normal colonic mucosa with colorectal cancer. Although the analysis indicated that the expression of Chk1 protein increases in about 50% of samples of colorectal cancer compared to normal mucosa, the observed changes were not statistically significant. Simultaneously relative amounts of phosphorylated Chk1 protein were found decreased in about 50% of studied cancer samples. Maybe, we did not observe significant changes in protein levels because Chk1 is essential for embryonic development, which suggests that Chk1 function is not only required upon genotoxic assaults but also under normal physiological conditions [18, 19]. Consistently with this, Chk1 protein was found to be phosphorylated in the absence of exogenous genotoxic agents (like UV or drugs such as hydroxyurea and aphidicolin), possibly due to signals generated by stalled replication forks occurring in each cell cycle. It is difficult at the present stage of studies to explain why the level of phosphorylated Chk1 protein is decreased in around 50% of studied cases. Little is known about the physiologic activities of Chk1 in the colorectal or its role in intracellular signaling. We are far from having a clear picture of how different signals converge at Chk1.
Chk1 and Chk2 prevent the cell cycle using similar mechanisms, namely inhibition of CDC25, but Chk2 protein has features that are not shared with Chk1. It has been demonstrated that expression of Chk2 protein is relatively stable and it is expressed at similar level in all phases of the cell cycle as well as in quiescent and terminally differentiated cells [35, 38]. Recent studies revealed that several Chk2 mutations had been shown to facilitate destabilization and increased degradation of Chk2 protein in human tumor [12, 38, 39]. These findings can explain our experimental observations, i.e., decreased amount of Chk2 protein in about 50% of studied cases. Mutant Chk2 protein was found to be less stable than wild type and could be expressed in various cell types only at a significantly reduced level than wild type [36]. Matsuoka et al. [36] have suggested that reduced expression of Chk2 may be an important inactivating mechanism, contributing to the development of lung cancer. To determine the potential role of Chk2 alterations in the pathogenesis of lymphoid neoplasm Tort et al. [25] have examined protein level in a series of tumors and non-neoplastic lymphoid samples. Chk2 protein level was found to be similar and independent of the proliferative activity of the tumors in all types of lymphomas and reactive lymphoid tissues. However, in one case of typical mantle cell lymphoma (MCL), two cases of blastoid MCLs, and two cases of large cell lymphomas, significant loss of protein expression including two samples with complete absence of Chk2 protein was shown. Also breast cancer studies revealed reduced Chk2 protein expression [27, 40]. Analyses of testicular germ-cell tumor demonstrated reduction and lack of protein expression in a subset of invasive neoplasms in contrast with the high protein level observed in other germ-cell tumors including all preinvasive lesions and normal germ cells, suggesting that decreased Chk2 protein expression may be involved in the progression of these neoplasms [38]. Similar results were observed by Bartkova et al. [41]. To determine whether Chk2 is activated in premalignant human tumors, they compared early, superficial lesions, early invasive and more advanced stages of urinary bladder cancer. Immunohistochemistry has shown heterogeneous positive staining in the early superficial lesions and earliest invasive stages, while more advanced primary carcinomas showed somewhat lower staining. The authors concluded that constitutive activation of the ATM-Chk2 pathway commonly occurs at preinvasive stages of major types of human tumors.
It is interesting that vulvae cancers did not exhibit loss of Chk2 expression at mRNA or protein level [23] and in gastric carcinoma expression of Chk2 protein is increased [24]. Shigeishi et al. [24] have suggested that expression of Chk2 protein increases in gastric tumors with mutant p53. However, Tort et al. [25] indicated that Chk2 protein expression alterations (in malignant lymphomas) occur independently of p53 status. Furthermore, Bartkova et al. [41] revealed that the ATM-Chk2-p53 cascade is activated in human bladder tumors before the occurrence of p53 mutations.
In our studies phosphorylated form of Chk2 was found to be abundant in normal mucosa and colorectal tumors. However, relative amounts of activated Chk2 appeared to be decreased in around 46% and increased in around 30% samples. It is interesting that the amount of pChk2 protein seems to increase in correlation with cancer progression into lymph nodes. The data of Ward and et al. [14] have shown that the kinase activity of Chk2 and number of Chk2 foci formed depend on the severity of DNA damage. These findings confirm that the DNA damage checkpoint pathway involving Chk2 protein might be inactivated in most of cancers, but exact cellular functions targeted by the Chk2 kinase is a challenge to be resolved by future studies.
Recently, Bartkova et al. [41, 42] have indicated that tumorigenic events which occur early in the progression of major human cancer types activate the ATR/ATM-regulated cell cycle checkpoints and thereby activate an inducible barrier against tumor progression and genetic instability. Activation of ATM-Chk2 pathway might limit the progression of lesions, possibly contributing to extended ‘latency’ periods or failure of early lesions to ever become malignant [41, 42].
The presented results confirm the significance of cell cycle checkpoint kinases 1 and 2 involvement in the process of neoplastic transformation. Nevertheless there are two faces of Chk1 and Chk2 in carcinogenesis. While reduced expression of Chk2 and pChk2 may be an important inactivating mechanism contributing to the development of colorectal neoplasm, an increasing expression seems to correlate with degree of malignancy.
References
Bartek J, Falck J, Lukas J (2001) Chk2 kinase—a busy messenger. Nat Rev Mol Cell Biol 2:877–886
Motoyama N, Naka K (2004) DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Deve 14:11–16
Parrilla-Castellar ER, Arlander SJH, Karnitz L (2004) Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair 3:1009–1014
Xu X, Tsvetkov LM, Stern DF (2002) Chk2 activation and phosphorylation-dependent oligomerisation. Mol Cell Biol 22:4419–4432
Bartek J, Lukas J (2001) Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol 13:738–747
Bartek J, Lukas J (2001) Pathways governing G1/S transition and their response to DNA damage. FEBS Lett 490:117–122
Busby EC, Leistritz DS, Abraham RT, Karnitz LM, Sarkaria JN (2000) The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res 60:2108–2112
Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ (2000) Chk1 is an essential kinase that is regulated by ATR and required for the G(2)/M DNA damage checkpoint. Genes Dev 14:1448–1459
Melo J, Toczynski D (2002) A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol 14:237–245
Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497–1501
Weiss RS, Matsuoka S, Elledge SJ, Leder P (2002) Hus1 acts upstream of chk1 in a mammalian DNA damage response pathway. Curr Biol 12(1):73–77
Ahn J, Urist M, Prives C (2004) The Chk2 protein kinase. DNA Repair 3:1039–1047
Lee CH, Chung JH (2001) The hCds (Chk2)-FHA domain is essential for a chain of phosphorylation events on hCds1 that is induced by ionizing radiation. J Biol Chem 276:30537–30541
Ward IM, Wu X, Chen J (2001) Threonine 68 of Chk2 is phosphorylated at sites of DNA strand breaks. J Biol Chem 276:47755–47758
Lukas J, Lukas C, Bartek J (2004) Mammalian cell cycle checkpoints: signaling pathways and their organization in space and time. DNA Repair 3:997–1007
Seo GJ, Kim SE, Lee YM, Lee JR, Hahn MJ, Kim ST (2003) Determination of substrate specificity and putative substrates of Chk2 kinase. Biochem Biophys Res Commun 304:339–343
Walworth NC (2001) DNA damage: Chk1 and Cdc25, more than meets the eye. Curr Opin Genet Dev 11:78–82
Chen Y, Sanchez Y (2004) Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair 3:1025–1032
Kalogeropoulos N, Christoforou Ch, Green AJ, Gill S, Ashcroft NR (2004) Chk-1 is an essential gene and is required for an S-M checkpoint during early embryogenesis. Cell Cycle 3:1196–1200
Stevens C, Smith L, La Thangue NB (2003) Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 5:401–409
Bertoni F, Codegoni AM, Furlan D, Tibiletti MG, Capella C, Broggini M (1999) CHK1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Genes Chromosomes Cancer 26:176–180
Menoyo A, Alazzouzi H, Espín E, Armengol M, Yamamoto H, Schwartz S (2001) Somatic Mutations in the DNA damage-response genes ATR and Chk1 in sporadic stomach tumors with microsatellite instability. Cancer Res 61:7727–7730
Reddy A, Yuille M, Sullivan A, Repellin C, Bell A, Tidy JA, Evans DJ, Farrell PJ, Gusterson B, Gasco M, Crook T (2002) Analysis of CHK2 in vulval neoplasia. Br J Cancer 86:756–760
Shigeishi H, Yokozaki H, Oue N, Kuniyasu H, Kondo T, Ishikawa T, Yasui W (2002) Increased expression of Chk2 in human gastric carcinomas harboring P53 mutations. Int J Cancer 99:58–62
Tort F, Hernandez S, Bea S, Martinez A, Esteller M, Herman JG, Puig X, Camacho E, Sanchez M, Nayach I, Lopez-Guillermo A, Fernandez PL, Colomer D, Hernandez L, Campo E (2002) CHK2-decreased protein expression and infrequent genetic alterations mainly occur in aggressive types of non-Hodgkin lymphomas. Neoplasia 100:4602–4608
Vahteristo P, Tamminen A, Karvinen P, Eeorola H, Eklund C, Aaltonen LA, Blomqvist C, Aittomaki K, Nevanlinna H (2001) p53, CHK2, and CHK1 genes in Finnish families with Li-Fraumeni syndrome: further evidence of CHK2 in inherited cancer predisposition. Cancer Res 61:5718–5722
Vahteristo P, Bartkova J, Eerola H, Syrjakoski K, Ojala S, Tamminen A, Kononen J, Aittomaki K, Heikkila P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H (2002) CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet 71:432–438
Kilpivaara O, Laiho P, Aaltonen LA, Nevanlinna H (2003) CHEK2 1100delC and colorectal cancer. J Med Genet 40:e110
Cybulski C, Gorski B, Huzarski T, Masojc B, Mierzejewski T, Debniak T, Teodorczyk U, Byrski T, Gronwald J, Matyjasik J, Złowocka E, Lenner M, Grabowska E, Nej K, Castaneda J, Medrek K, Szymanska A, Kurzawski G, Suchy J, Oszurek O, Witek A, Narod SA, Lubinski J (2004) CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet 75(6):1131–1135
Weischer M, Bojesen SE, Tybjoerg-Hansen A, Axelsson CK, Nordestgaard BG (2006) Increased risk of breast cancer associated with CHEK2* 1100delC. J Clin Oncol 24:1–7
Cadman E, Bastwick IB, Eichberg I (1997) Determination of protein by a modified Lowry procedure in the presence of some commonly used detergents. Anal Biochem 96:21–23
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–688
Glass WF, Briggs RC, Hnilica LC (1981) Identification of tissue-specific nuclear antigens transferred to nitrocellulose from polyacrylamide gel. Science 211:70–72
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
Lukas C, Bartkova J, Latella L, Falck J, Mailand N, Schroeder T, Sehested M, Lukas J, Bartek J (2001) DNA Damage-activated kinase Chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res 61:4990–4993
Matsuoka S, Nakagawa T, Masuda A, Haruki N, Elledge SJ, Takahashi T (2001) Reduced expression and impaired kinase activity of a Chk2 mutant identified in human lung cancer. Cancer Res 61:5362–5365
Semba S, Ouyang H, Han SY, Kato Y, Horii A (2000) Analysis of the candidate target genes for mutation in microsatellite instability-positive cancers of the colorectum, stomach and endometrium. Int J Oncol 16:731–737
Bartkova J, Falck J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J (2001) Chk2 tumour suppressor protein in human spermatogenesis and testicular germ-cell tumours. Oncogene 20(41):5897–5902
Wu X, Webster SR, Chen J (2001) Characterization of tumor-associated Chk2 mutations. J Biol Chem 274:2971–2974
Sullivan A, Yuille M, Repellin C, Reddy A, Reelfs O, Bell A, Dunne B, Gusterson BA, Osin P, Farrell PJ, Yulug I, Evans A, Ozcelik T, Gasco M, Crook T (2003) Concomitant inactivation of p53 and Chk2 in breast cancer. Oncogene 21(9):1316–1324
Bartkova J, Hořejši Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864–870
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou L-VF, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Ørntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VS (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444:633–637
Acknowledgements
This project was supported by grant No. 505/446 from the University of Lodz, Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Magdalena Stawinska and Adam Cygankiewicz contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stawinska, M., Cygankiewicz, A., Trzcinski, R. et al. Alterations of Chk1 and Chk2 expression in colon cancer. Int J Colorectal Dis 23, 1243–1249 (2008). https://doi.org/10.1007/s00384-008-0551-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0551-8