Abstract
Background and aims
Microsatellite instability (MSI) is a potential indicator of prognosis in patients with colorectal cancer (CRC). To date, there are a limited number of studies which investigated its role in advanced CRC. Our study investigated the value of high degree of MSI (MSI-H) in patients treated with 5-FU/oxaliplatin-based chemotherapy which has been done by only one further study recently.
Patients and methods
In this study, we investigated tumour tissues from 108 patients with metastatic CRC who were treated in a prospective, randomised trial comparing two oxaliplatin and 5-FU-based therapy regimens (FUFOX vs. CAPOX) involving a total of 474 patients. We determined the incidence and prognostic value of a high degree of microsatellite instability. The specimens were analysed by PCR corresponding to the National Institute of Health reference panel. In addition, immunostaining of the mismatch repair proteins MLH1, MSH2 and MSH6 was performed.
Results and findings
The incidence of MSI-H was 4%. MSI-H was correlated with a lower rate of disease control compared to non-MSI-H patients (p = 0.02). However, there was no correlation between MSI-H and progression-free survival or overall survival.
Interpretation and conclusion
MSI-H incidence in metastatic CRC was low. Our data suggest that MSI-H may be correlated with a poorer response to a 5-FU/oxaliplatin treatment. This finding needs confirmation in a larger cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an estimated incidence of 1,023,000 new cases annually, colorectal cancer (CRC) represents the fourth most common malignant solid tumour worldwide [1]. About 20–25% of patients present metastatic disease at the time of initial diagnosis. In addition, up to 50% of patients with curatively resected CRC will develop metachronous metastatic disease. Palliative chemotherapy has shown to improve overall survival (OS) and quality of life. To date, several chemotherapy schemes including infusional and oral 5-fluorouracil (5-FU), irinotecan and oxaliplatin can be applied. Recently, targeted therapies with monoclonal antibodies against the vascular endothelial growth factor and the epidermal growth factor receptor have also become available [2, 3]. This increase of active drugs enables the choice between numerous therapeutic options. However, reliable prediction of tumour response, prognosis and toxicity for each therapy scheme for an individual patient is presently not yet available. Consequently, molecular markers which may predict the clinical course or toxicity of a certain patient may guide treatment choices.
One of these potential prognostic factors is high-level microsatellite instability (MSI-H). MSI-H is related to a deficient mismatch repair (MMR) system [4, 5]. Such a defect derives from a somatic hereditary mutation of a MMR gene or from hypermethylation of the MLH1 promotor. Germline mutations in MMR genes in the context of Lynch syndrome account for 2–5% of CRC [6]. An additional 12–15% of CRC display MLH1 promoter hypermethylation [7–11]. MSI-H CRC have characteristic clinical and histopathological features. In particular, MSI-H CRC has a favourable prognosis compared to non-MSI-H CRC [12]. However, the prognostic value of MSI-H has been determined mainly in surgical cases. The value of MSI-H in surgically resected colon cancers and adjuvant 5-FU-based chemotherapy remains controversial. While earlier studies suggested a benefit from adjuvant chemotherapy for MSI-H CRC [13, 14], more recent studies did not find a benefit from adjuvant chemotherapy for MSI-H CRC compared to surgery only [15–18]. The latter studies are in line with in vitro studies regarding chemoresistance to 5-FU for MSI-H CRC cell lines [19, 20]. In addition, Arnold et al. demonstrated an increased chemosensitivity after demethylation treatment in CRC cell lines with MLH1 promoter hypermethylation [21].
The data regarding the value of MSI-H in metastatic CRC undergoing 5-FU-based palliative chemotherapy are very limited. The results from four studies suggested mainly a benefit from 5-FU-based palliative chemotherapy [22–25].
Most recently, des Guetz et al. reported for the first time upon the prognostic value of MSI-H in patients treated with palliative first-line combination chemotherapy with 5-FU/oxaliplatin (FOLFOX) in 44 patients with metastatic CRC [26]. The authors did not find a significant difference between progression-free survival (PFS), OS and response rates (RR) between MSI-H and microsatellite stable (MSS) cases in this small series of patients not included in a clinical trial.
In vitro data suggest a chemoresistance of cancer cell lines with MMR deficiency upon treatment with cisplatin and carboplatin but not upon oxaliplatin [27]. These results were later confirmed by the same group using a xenograft model [28].
To further evaluate the prognostic and predictive value of MSI-H in metastatic CRC treated with 5-FU/oxaliplatin-based first-line chemotherapy, we investigated 108 tumour samples from patients included in a randomised prospective trial. We first aimed to investigate the incidence of MSI-H by performing microsatellite analysis and immunostaining of the MMR proteins MLH1, MSH2 and MSH6. Secondly, MSI-H was correlated to clinical data to evaluate its predictive and prognostic value for tumour response and PFS and OS.
Materials and methods
Study design
Tissue samples were derived from patients with metastatic CRC participating in a prospective randomised phase III first-line chemotherapy trial of the AIO Group (Arbeitsgemeinschaft Internistische Onkologie of the German Cancer Society) [29]. A total of 474 patients were randomised to be treated with either 5-FU/folinic acid (FA) and oxaliplatin (FUFOX: oxaliplatin, 50 mg/m2; FA, 500 mg/m2; continuous 5-FU, 2,000 mg/m2/22 h; on day 1, 8, 15, 22; q day 36; n = 51) or capecitabine and oxaliplatin (CAPOX: oxaliplatin, 70 mg/m2 on day 1 and 8; capecitabine, 2 × 1,000 mg/m2/day consecutively for 2 weeks, q day 22; n = 52). Eligibility and exclusion criteria are published elsewhere [29]. The study was approved by the ethical committee of the Central Hospital Bremen and of the Medical Faculty of the Ruhr-University Bochum.
Clinically, the combination of capecitabine and oxaliplatin showed no significant difference in terms of RR, PFS (as the primary endpoint) and OS when compared to 5-FU/FA/oxaliplatin. Porschen et al. found an overall RR of 48% in the CAPOX arm and 54% in the FUFOX arm (p = 0.7). The median PFS was 7.1 months (CAPOX) and 8.0 months (FUFOX, p = 0.117) and the median OS was 16.8 months (CAPOX) versus 18.8 months (FUFOX, p = 0.26). These data are consistent with the findings of other trials [30, 31]. Moreover, no clinical factor was found to be predictive for definition of a subgroup of patients benefiting more or less from each fluoropyrimidine backbone [29].
Patient characteristics
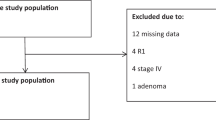
This analysis includes a subgroup of 108 patients (23% of all patients) from the clinical trial, recruited from July 2002 to July 2004, of whom tumour tissue were available. The median follow-up time was 16.5 months (range, 1–37 months). Patients’ characteristics were: 62 male, 42 female, with a median age of 64.7 (range 38–81) years. Fifty-seven patients had synchronous metastases, and 43 had metachronous metastases. In four patients, the date of appearance of metastasis was not recorded.
As expected in metastatic CRC (mCRC) cohorts, one third of patients had primarily rectal cancer whereas the primary tumour of the majority was in the colon (Table 1). The investigated tissue samples included primary tumours (n = 84) as well as metastases (n = 8). In 12 patients, the origin of the tissue was not recorded.
Immunohistochemistry
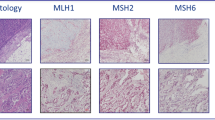
Immunostaining and interpretation were performed as described previously [32]. As primary antibodies, mouse monoclonal antibodies for MLH1 (BD Biosciences, 1:20), MSH2 (BD Biosciences, 1:20) and MSH6 (BD Biosciences, 1:50) were applied. Staining was considered only informative when there was normal nuclear staining in adjacent non-neoplastic cells, which served as positive internal control. The part of positive stained nuclei was determined semi-quantitatively by light microscope and classified as follows: <1% represented a lack of expression of the MMR proteins; 1–10% showed a reduction of expression; >10% indicated a regular expression. Immunohistochemistry of MLH1 and MSH2 has a sensitivity of 92% to 95% and specificity of 99% to 100% to detect MSI-H [33–35].
Microsatellite analysis
Tumour and normal tissue were microdissected by a skilled pathologist. DNA was isolated with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Microsatellite–polymerase chain reaction (PCR) reactions were performed as described previously [32]. To screen for microsatellite instability (MSI), all specimens were investigated with the microsatellite marker Bat-26 first. Cases with instability of Bat-26 were tested with the complete National Institute of Health reference marker panel (Bat-25, D5S346, D17S250, D2S123) [36]. Tumour tissue without normal tissue available (n = 3) was exclusively investigated with Bat-26 and Bat-25. The exclusive application of Bat-26 has shown a sensitivity for detection of MSI-H of about 95% [37]. Tumours were classified as MSI-H if at least two of five markers showed instability. MSI-L was defined if instability was evident in only one marker. Tumours were classified as MSS in cases where no marker was instable.
Statistical analyses
Fisher’s exact test was used to evaluate the associations between MSI and other dichotomous variables. Student’s T-test was performed to calculate the relationship between continuous variables and dichotomous variables. Significant differences between a continuous variable and different groups were identified by the Kruskal–Wallis test. The Kaplan–Meier method was used to determine the OS and the PFS [38]. The OS and the PFS of the subgroups (MSI-H positive vs. MSI-H negative) were compared by the log-rank test. All tests were two sided. For all tests, p values <0.05 were considered as significant.
Results
Incidence of microsatellite instability
Of the 108 patients included in this study, 89 (83%) tumours were analysable by PCR-based MSI testing. Additional 15 cases were evaluable by immunohistochemistry of MLH1, MSH2 and MSH6. Tissue samples from four (4%) patients were not analysable due to low DNA quality and staining artefacts (no staining of normal tissue). Among 104 analysable tumours, we identified four (4%) MSI-H tumours and one (1%) MSI-L tumour. One tumour exhibited a reduction of MSH6 while displaying MSS. The latter two cases were analysed within the MSS group.
Correlation between MSI and clinicopathological variables
Four (8%) out of 51 patients treated with FUFOX had a MSI-H tumour, while no patient out of 53 treated with CAPOX had a MSI-H tumour (p = 0.054) MSI-H was not correlated to any other clinicopathological variables recorded (age, sex, localisation, syn- vs. metachronous metastatic disease) (data not shown).
Correlation between MSI and remission status
Tumour response evaluation yielded that one of four MSI-H-positive patients had a WHO-classified complete remission and one more patient had a stable disease whereas two (50%) patients showed progressive disease. Among 100 non-MSI-H tumours, three (3%) patients had a complete remission and 52 patients (52%) had a partial remission. Thirty-one (31%) patients exhibited a stable disease and four (4%) had disease progression. No significant relation could be found with regards MSI-H status when comparing patients with objective response (PR + CR) to patients with stable disease or progressive disease (p = 0.30).
In contrast, MSI-H-positive patients compared with non-MSI-H patients actually showed a significant disadvantage considering the combined outcome marker with disease control (defined as PR, CR or SD) versus progressive disease (PD). Disease control was observed for 50% of MSI-H-positive and 95.6% of non-MSI-H patients (p = 0.019). PD appeared in 50% of patients with MSI-H-positive and 4.4% of patients with non-MSI-H tumours (Table 2).
Correlation between MSI and progression-free survival and overall survival
This subcohort of patients, representing approximately 25% of all patients included in the clinical trial, had a median PFS of 7.8 months (95% CI, 5.8–9.8 months) and a median OS of 18.9 months (95% CI, 15.8–22.0 months) which were not significantly different from the data from the whole clinical trial [29]. PFS and OS were significantly better in patients with metachronous metastases as compared to patients with synchronous metastases (Table 3). The other patient characteristics did not demonstrate any relation to PFS or OS, respectively.
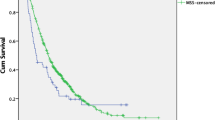
During the follow-up time, 88 patients showed disease progression of which three patients had an MSI-H and one patient an MSI-L tumour. Sixteen patients, including one patient with an MSI-H tumour, had no disease progression at last follow-up. The median PFS was 7.9 months for non-MSI-H patients (95% CI, 6.1–9.74 months) and 2.5 months for patients with MSI-H tumours (95% CI, 0.9–4.1 months) (p = 0.59) (Table 3; Fig. 1).
At the time of last follow-up, 69 patients died, including one patient with MSI-H tumour, one patient with MSI-L tumour and 67 with non-MSI-H tumours. Thirty-five patients were alive, including three patients with MSI-H tumours (75%) and 32 patients with non-MSI-H tumours. The median OS for patients with non-MSI-H tumours was 18.9 months (95% CI, 15.7–22.2 months). The MSI-H-positive patients did not reach the median during the follow-up. However, the difference was not statistically significant for OS (p = 0.13) (Table 3; Fig. 2).
Discussion
So far, only a few studies investigated the incidence of MSI-H in metastatic CRC and its correlation to prognosis and treatment response. We therefore analysed data from a cohort of 25% patients included into a prospective randomised trial treated with oxaliplatin in combination with intravenous versus oral 5-FU, with respect to the incidence and prognostic and predictive value of MSI-H.
Incidence of microsatellite instability
The incidence of MSI-H in unselected CRC is reported to range from 12–18% [36]. The incidence of MSI-H in our study was 4%. Our data are consistent with most previously published data regarding metastatic CRC [39, 40]. However, some studies reported a markedly higher incidence of MSI in metastatic CRC [24, 41, 42]. The discrepancy of some studies with a higher incidence of MSI to our data might be a result of a variable definition of MSI-L/MSI-H, use of different markers or a consequence of selection of patients. The inclusion criteria of patients for the prospective clinical trial may have caused some selection bias, since only patients with ECOG 0–1 performance status were eligible for the trial. In addition, we preferentially analysed tumour tissue from the primary colorectal cancer (n = 84) rather than the metastases. However, this fact was unlikely to cause a bias since MSI-H is an early event in colorectal carcinogenesis.
Correlation of MSI-H and prognostic variables
Only a few studies have addressed the prognostic value of MSI-H in metastatic CRC treated with 5-FU-based chemotherapy. Liang et al. detected a significant favourable outcome of MSI-H patients treated with 5-FU-based chemotherapy (n = 169), regarding response rate (65.7% vs. 35.1%; p = 0.001) and median OS (24 month vs. 13 month; p = 0.0001), when compared to non-MSI-H patients [23]. For patients without chemotherapy (n = 75), there was no correlation between MSI status and survival. The authors concluded that the predictive value might be explained by a higher chemosensitivity of MSI-H-positive tumours but not by a lower aggressiveness of tumour growth. In addition, Brueckl et al. observed a significant better median OS for patients treated with a 5-FU-based chemotherapy (33 months vs. 19 months, p = 0.021) and a higher remission rate (72% vs. 41%; p = 0.072) for MSI-H-positive patients (n = 7) compared to non-MSI-H patients (n = 36) [22].
The first study which reported the correlation of MSI-H and the outcome of combination chemotherapy with FOLFOX 4 or FOLFOX6 first-line palliative chemotherapy in 44 patients metastatic CRC was published while we were conducting our analyses [26]. The authors did not find a significant difference between PFS, OS and RR between MSI-H (n = 9) and MSS (n = 31) cases. However, the limited sample size (n = 44) has to be considered. In addition, it has to be noted that patients were not treated within a controlled trial with defined inclusion and exclusion criteria which may have introduced unintended bias.
In our study, we did not observe a favourable or unfavourable prognosis of MSI-H-positive patients compared to non-MSI-H patients with respect to PFS and OS. However, our analysis must be interpreted bearing in mind the very low number of MSI-H cases.
Considering disease control (defined as CR, PR and SD) versus PD, we found a significant disadvantage of MSI-H-positive patients compared to non-MSI-H patients. If this finding could be confirmed in a larger set of patients, these findings differ from previous studies analysing 5-FU-based protocols and may reflect a possible unfavourable outcome for oxaliplatin administration for this subgroup.
However, if the low number of MSI-H-positive patients is consistent in other prospective mCRC trials, the use of this marker is of limited value. Furthermore, other potential prognostic markers such as p53, SMAD4, thymidylate synthetase and K-ras mutations should be included in multivariate analyses.
References
Ferlay J, Bray F, Pisani P et al (2004) GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC CancerBase no. 5, version 2.0. IARC, Lyon, France
Kelly HGoldberg RM (2005) Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol 23:4553–4560
Meyerhardt JAMayer RJ (2005) Systemic therapy for colorectal cancer. N Engl J Med 352:476–487
Jiricny JNyström-Lahti M (2000) Mismatch repair defects in cancer. Curr Opin Genet Dev 10:157–161
Grady WM (2004) Genomic instability and colon cancer. Cancer Metastasis Rev 23:11–27
Lynch HT, Boland CR, Rodriguez-Bigas MA et al (2007) Who should be sent for genetic testing in hereditary colorectal cancer syndromes? J Clin Oncol 25:3534–3542
Thibodeau SN, French AJ, Cunningham JM et al (1998) Microsatellite instability in colorectal cancer: different mutator phenotypes and the principial involvement of hMLH1. Cancer Res 58:1713–1718
Cunningham JM, Christensen ER, Tester DJ et al (1998) Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 58:3455–3460
Kane MF, Loda M, Gaida GM et al (1997) Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 57:808–811
Veigl ML, Kasturi L, Olechnowicz J et al (1998) Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A 95:8698–8702
Herman JG, Umar A, Polyak K et al (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A 95:6870–6875
Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609–618
Hemminki A, Mecklin JP, Jarvinen H et al (2000) Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 119:921–928
Elsaleh H, Joseph D, Grieu F et al (2000) Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 355:1745–1750
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257
Carethers JM, Smith EJ, Behling CA et al (2004) Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 126:394–401
Jover R, Zapater P, Castells A et al (2006) Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 55:848–855
Benatti P, Gafa R, Barana D et al (2005) Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res 11:8332–8340
Carethers JM, Chauhan DP, Fink D et al (1999) Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 117:123–131
Tajima A, Hess MT, Cabrera BL et al (2004) The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance. Gastroenterology 127:1678–1684
Arnold CN, Goel A, Boland CR (2003) Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer 106:66–73
Brueckl WM, Moesch C, Brabletz T et al (2003) Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res 23:1773–1777
Liang JT, Huang KC, Lai HS et al (2002) High-frequency microsatellite instability predicts better chemosensitivity to high-dose 5-fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer 101:519–525
Chen WS, Chen JY, Liu JM et al (1997) Microsatellite instability in sporadic-colon-cancer patients with and without liver metastases. Int J Cancer 74:470–474
Kochhar R, Halling KC, McDonnell S et al (1997) Allelic imbalance and microsatellite instability in resected Duke's D colorectal cancer. Diagn Mol Pathol 6:78–84
des Guetz G, Mariani P, Cucherousset J et al (2007) Microsatellite instability and sensitivity to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res 27:2715–2719
Fink D, Nebel S, Aebi S et al (1996) The role of DNA mismatch repair in platinum drug resistance. Cancer Res 56:4881–4886
Fink D, Zheng H, Nebel S et al (1997) In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res 57:1841–1845
Porschen R, Arkenau HT, Kubicka S et al (2007) Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol 25:4217–4223
Diaz-Rubio E, Tabernero J, Gomez-Espana A et al (2007) Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol 25:4224–4230
Cassidy J, Clarke S, Diaz-Rubio E et al (2007 ASCO Annual Meeting Proceedings) XELOX compared to FOLFOX4: Survival and response from XELOX-I/NO16966, a randomized phase III trial of first-line treatment for metastatic colorectal cancer (MCRC). J Clin Oncol 25(18s) (suppl; abstr 4030)
Kunstmann E, Vieland J, Brasch FE et al (2004) HNPCC: six new pathogenic mutations. BMC Med Genet 5:16
Dietmaier W, Wallinger S, Bocker T et al (1997) Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 57:4749–4756
Lindor NM, Burgart LJ, Leontovich O et al (2002) Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 20:1043–1048
Ruszkiewicz A, Bennett G, Moore J et al (2002) Correlation of mismatch repair genes immunohistochemistry and microsatellite instability status in HNPCC-associated tumours. Pathology 34:541–547
Boland C, Thibodeau S, Hamilton S et al (1998) A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Zhou XP, Hoang JM, Li YJ et al (1998) Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosomes Cancer 21:101–107
Kaplan EMeier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 158:23–39
Gonzalez-Garcia I, Moreno V, Navarro M et al (2000) Standardized approach for microsatellite instability detection in colorectal carcinomas. J Natl Cancer Inst 92:544–549
Lothe RA, Peltomaki P, Meling GI et al (1993) Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 53:5849–5852
Jernvall P, Makinen MJ, Karttunen TJ et al (1999) Microsatellite instability: impact on cancer progression in proximal and distal colorectal cancers. Eur J Cancer 35:197–201
Evertson S, Wallin A, Arbman G et al (2003) Microsatellite instability and MBD4 mutation in unselected colorectal cancer. Anticancer Res 23:3569–3574
Acknowledgements and grant support
This study was supported in part by a grant of the German Cancer Aid (Deutsche Krebshilfe, 70–3033-Schm 4) to WS and an intramural research grant of the Medical Faculty of the Ruhr-University Bochum to KS. We thank Sabine Geiger, Hedi Safa, Sandra Grasediek and Britta Redeker for technical assistance and the AIO for providing clinical data. We thank the participating pathologists for providing tissue blocks. The authors state to have no financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. I. Müller and K. Schulmann contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Müller, C.I., Schulmann, K., Reinacher-Schick, A. et al. Predictive and prognostic value of microsatellite instability in patients with advanced colorectal cancer treated with a fluoropyrimidine and oxaliplatin containing first-line chemotherapy. A report of the AIO Colorectal Study Group. Int J Colorectal Dis 23, 1033–1039 (2008). https://doi.org/10.1007/s00384-008-0504-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0504-2