Abstract
Background
Calcitonin-gene-related peptide (CGRP) and substance P (SP) are neurotransmitters of extrinsic primary afferent neurons located within the dorsal root ganglia. In experimental models of colitis in rats and rabbits, a protective role of SP and CGRP on intestinal mucosa was presumed. The mucosal protection partly depends on a CGRP-mediated modulation of mucosal blood flow. Limited data are available regarding CGRP- or SP-mediated effects on epithelial cell restitution. Having shown earlier that SP-stimulated fibroblasts but not CGRP-stimulated fibroblasts induce epithelial cell migration in vitro, the aim of this study was to explore whether mast cells mediate effects of SP and CGRP on epithelial cell restitution in vitro.
Methods
Mast cells (C57) were exposed to SP [10−12–10−6 M] and CGRP [10−12–10−7 M]. After a 24-h incubation period, the cell supernatants (conditioned media, CDM) were taken from mast cell cultures and directly applied to rat intestinal epithelial cell lines-18 or Caco-2 monolayers, which had been wounded with a razor blade 24 h prior to the experiments. Epithelial cell migration was assessed by counting cells across the wound edge and epithelial cell proliferation was measured using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide test.
Results
CGRP significantly induced epithelial cell migration and proliferation via mast cells when supernatants were directly applied to epithelial cells in vitro. The effects on epithelial cell migration were abolished after neutralizing anti-transforming growth factor-beta (TGF-β) had been added to the cell cultures. SP had no effects on epithelial cells following stimulation of mast cells.
Conclusion
CGRP modulates epithelial cell restitution in vitro mediated by mast cells. The CGRP- and mast-cell-induced epithelial cell migration is TGF-β dependent. This observation underlines an important role for extrinsic primary afferent neurons in mucosal defence and repair and in keeping the mucosal homeostasis. This knowledge leads to a better understanding of the interaction of the enteric nervous system and wound healing and may, in the future, lead to new therapeutic approaches to inflammatory diseases of the intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the last decades a chain of evidence has been gained that extrinsic primary afferent neurons located within the dorsal root ganglia protect the gastrointestinal mucosa against injury. The complete ablation of the extrinsic afferent nervous system by the neurotoxin capsaicin worsened mucosal damage in experimental models of colitis in rats [1–3] and the stimulation of afferent nerve endings of the mucosa immediately before ethanol- or aspirin-induced injury significantly reduced mucosal damage in the rat stomach [4–6]. Similarly, stimulation of afferent nerve endings protected colonic mucosa in the acetic-acid-induced experimental model of colitis in rats [7]. While capsaicin ablates the extrinsic afferent nervous system, it also influences pepdidergic nerves of the intrinsic nervous system.
The mechanism leading to protection presumably is the exocytotic release of neurotransmitters, mainly calcitonin-gene-related peptide (CGRP), at both central and peripheral endings of primary afferent neurons [8]. In the event of mucosal injury, an early decrease of colonic contents of CGRP was shown for the rabbit immune complex/formalin-induced colitis [9] and for the trinitrobenzene sulfonic acid/ethanol-induced experimental colitis in rats [2]. CGRP is a potent vasodilatator [10] and the protective effect of extrinsic afferent neurons was thought to be mediated by a CGRP-induced increase in mucosal blood flow [11].
We showed earlier that stimulation of primary afferents in the rat colon by capsaicin induced an increase in epithelial cell nucleotide turnover and presumably in mucosal epithelial cell proliferation. This effect was abolished by sensory desensitisation or application of specific antagonists to CGRP or substance P (SP) [12]. This observation indicated a possible modulation of epithelial cell function by the sensory neurotransmitters CGRP and SP. Although the human colon-cancer-derived epithelial cell lines CaCo-2 and HT-29 express neurokinin (NK)-1 receptors after pre-treatment with pro-inflammatory cytokines [13], we did not detect any direct effects of SP or CGRP on non-conditioned CaCo-2 cells or rat intestinal epithelial cell lines (IEC)-18 in an in vitro wounding model [14]. It is of interest that, using the same model, a transforming growth factor beta (TGF-β)-mediated induction of epithelial cell migration and a significant inhibition of epithelial cell proliferation mediated by fibroblasts was induced by SP but not by CGRP [14].
In addition to the possible direct interaction of sensory neurotransmitters with epithelial cells and fibroblasts, a CGRP-induced deliberation of mast cells was reported previously [8]. Mast cells are known as key elements in type I hypersensitivity but within the last decades they have become of interest as they are also involved in inflammatory processes such as inflammatory bowel disease, where they are markedly increased [15]. Mast cells express a great number of cytokines including interleukins (IL-1, IL-3, IL-4, IL-5 and IL-6), colony-stimulating factors like granulocyte macrophage colony-stimulating factor, interferon-γ or growth-factor-responsive genes that code for small secreted glycoproteins with cytokine-like properties as macrophage inflammatory protein (MIP1-alpha and MIP1-beta), TGF-β and tumor necrosis factor-α [16–18].
The aim of our present study therefore was to elucidate possible mast-cell-mediated effects of sensory neurotransmitters on epithelial cell restitution in vitro.
Material and methods
Cell lines, cell cultures and CDM
Rat intestinal epithelial cell lines (IEC-18) and the human-carcinoma-derived cell line Caco-2 were purchased from American Type Culture Collection, Rockville, MD, USA. The mast cells used (Cl.MC/C57.1) were a growth-factor-independent murine mast cell line. This cell line was originally derived from bone-marrow-cultured mast cells isolated from C57BL/6J mice and subsequently cloned by dilution (kind gift of SJ Galli, Stanford, CA, USA). All cells were routinely grown in Dulbecco’s modified eagle medium (DMEM) containing 5% (IEC-18) or 10% (Caco-2) fetal calf serum (FCS).
Cells were stored under 5% CO2 atmosphere at 37°C. 3-[4, 5-Dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT) was used for the assessment of epithelial cell proliferation by colorimetric analysis. TGF-β antibody was purchased from R&D Systems Inc., Minneapolis, MN, USA. SP and CGRP were purchased from Sigma Chemical Co., St. Louis, MO, USA. Supernatants of mast cells were taken from subconfluent monolayers after a 24-h time period in serum-deprived media and another 24-h incubation period with different concentrations of SP [10−6, 10−9 and 10−12 M] and CGRP [10−7, 10−9 and 10−12 M].
Measurements of the effects of SP- and CGRP-stimulated mast cell media on epithelial cell proliferation
All experiments were performed as described previously [19, 20]. In brief, IEC-18 and Caco-2 cells were seeded into 24-well plates (5 × 104 cells/well) in the presence of DMEM containing 5% FCS. Culture media were changed 24 h before experiments to serum-deprived media (containing 0.1% FCS in DMEM). Proliferation of epithelial cells was assessed after SP- and CGRP-stimulated mast cell supernatants were added to media (20%). Supernatants of non-treated mast cells served as controls. After 24 h (48 h), cells were photographed and counted. Proliferation assays were evaluated using MTT as described previously [20, 21]. In brief, 2 mg/ml of MTT was added to the media followed by an incubation period of 4 h. Cells were washed and consecutively dissolved with dimethyl sulfoxide. Quantified measuring of absorbance at 550 nm (reference wavelength, 690 nm) was performed using a microplate reader (EL×800G, BIO-TEK Instruments, Inc., VT, USA). Experiments for all substances were performed at least in quadruplicate.
In vitro wound assays
Confluent monolayers of IEC-18 and Caco-2 cells in 100 × 15-mm petri dishes (Falcon®, Becton Dickinson Labware, NJ, USA) were wounded with a razor blade; two wounds were made approximately 10–15 mm across the dish, separated by about 1.5 cm. Afterwards, cells were washed with phosphate-buffered saline and subsequently cultured for 24 h in fresh serum-deprived medium (0.1% FCS). Chemically, conditioned media (CDM) [20%] was added to the media. A computer-based microscopy imaging system (Axiovision®, Carl Zeiss, Munich, Germany) was used for the determination of wound healing at 0 h (Carl Zeiss®, Axiovert 25 microscope at 400-fold magnification) and 24 h later. Identification of migration was assessed by quantification of the number of cells observed across the wound, compared to the same frame at 0 h. Several wound areas (at least five) per plate were investigated to quantify migration. To verify possible TGF-β-mediated effects, monolayers of IEC-18 and Caco-2 cells were treated with anti-TGF-β1 (5 ng/ml) and CDM [20%]. To exclude possible proliferative effects that interfered with the migration of epithelial cells, mitomycin was added to the media to prevent proliferation while epithelial cell migration was assessed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± standard error of the mean (SEM). Differences between the groups were tested with analysis of variance and Wilcoxon test. Values lower than p = 0.05 were considered statistically significant.
Results
Epithelial cell proliferation
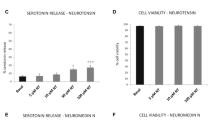
Supernatants (= conditioned media, CDM) of CGRP-treated C57 mast cells [20% = 1/5 of supernatant] induced a significant proliferation in IEC-18 cells and Caco-2 cells after 24 h (Fig. 1). This effect was observed in both cell lines in a dose-dependent manner (CGRP [10−12 > 10−9 > 10−7 M]. Supernatants of SP-stimulated mast cells had no effect on migration or proliferation in both IEC-18 and Caco-2 cells as CGRP alone on IEC-18 cells and Caco-2 cells (data not shown).
Proliferation of IEC-18 and Caco-2 cell incubated with 50 μl mast cell media [10% CDM] stimulated with CGRP [10−12–10−7 M]—IEC-18 and Caco-2 cells were incubated with CDM. One incubation with CDM without CGRP is also presented as control. After a 24-h incubation period, cell proliferation was measured with the MTT assay. The results represent means ± SEM of 12 separate experiments. Asterisks show a significant induction of proliferation by incubation of the cells (* = p < 0.05, *** = p < 0.01 vs. control)
Epithelial cell migration
CDM of CGRP-treated mast cells [20% and 10%] induced a significant increase of migrating cells in the rat small intestine-derived cells (IEC-18) and in the human colon-cancer-derived Caco-2 cells (Figs. 2 and 3 [10% CDM]; data for 20% CDM are not presented but similar). While IEC-18 cells showed migration in a dose-dependent manner, the migration rate of Caco-2 cells showed no strict dose-dependent effect at higher CGRP concentrations. Again, SP-treated mast cells had no effect on epithelial cell migration (data not shown).
Effect of 50 μl CDM mast cell media (not stimulated vs. stimulated with CGRP [10−9 M] on cell migration in Caco-2 cell monolayers—Caco-2 cells were grown in monolayers and injured with a razor blade cut, indicated by the arrow). Following wounding, the cells were cultured for 24 h in fresh serum-deprived medium. The cell preparation was then incubated with designated CDM (upper panel 50 μl conditioned unstimulated mast cell media, lower panel stimulated with CGRP [10−9 M]). After 24-h incubation period, the cell migration across the wound edge was counted
Effect of CDM [10%] stimulated with CGRP [10−12–10−7 M] on cell migration in IEC-18 and Caco-2 cell monolayers—IEC-18 cells and Caco-2 cells were incubated with CDM/CGRP [10−12–10−7 M] to explore effects on migration. After a 24-h incubation period, the cell migration across the wound edge was measured. The results shown represent means ± SEM of at least eight separate experiments. Asterisks show a significant induction of migration of the cells (* = p < 0.05, *** = p < 0.01 vs. control)
Stimulation with a lower concentration of CGRP-treated mast cells CDM showed similar effects on Caco-2 cells while the effect on IEC-18 cells was markedly increased depending on the CGRP concentration used (Fig. 3). Using mitomycin in our experiments to suppress possible proliferative effects did not alter the result of a significant migration (data not presented) indicating that the observed effects more likely represent migration rather than proliferation.
Inhibition of epithelial cell migration with neutralizing TGF-β1 antibodies
Addition of neutralizing TGF-β1 antibodies to wounded IEC-18 and Caco-2 monolayers that were stimulated with media of CGRP-conditioned mast cells significantly reduced the number of migrating cells to the level of the control group. This reduction was significant compared to CDM CGRP [10−9 M] (Fig. 4) and detectable in both cell lines.
Effect of CDM [10%] stimulated with CGRP [10−9 M] and TGF-β1 antibody on cell migration in IEC-18 and Caco-2 cell monolayers—IEC-18 cells and Caco-2 cells were incubated with CDM/CGRP [10−9 M] and TGF-β1 antibody to explore possible TGF-β effects on migration. After a 24-h incubation period, the cell migration across the wound edge was measured. The results shown represent means ± SEM of 12 separate experiments. Asterisks show a significant induction of migration of the cells (* = p < 0.05 vs. control)
Discussion
CGRP modulates epithelial cell function in vitro. This effect is mediated by CGRP-stimulated mast cells, as the media of CGRP-conditioned mast cells induced a significant increase in epithelial cell proliferation and migration in our in vitro model. These results indicate that CGRP might be an important factor in the modulation of epithelial cell restitution in vivo and in keeping the mucosal homeostasis, although until today there are not sufficient data available in humans.
CGRP is a neurotransmitter of primary extrinsic afferent neurons [8] which is released from neuroeffector junctions into the surrounding tissues following mucosal injury [2, 9]. Primary extrinsic afferents have been shown to protect the mucosa against injury in various animal models [1, 2, 9]. So far, the protective effect was believed to be mediated by a CGRP-induced vasodilatation and modulation of mucosal blood flow [10]. To our knowledge, this is the first report showing an effect of CGRP on epithelial cell restitution mediated by mast cells. Epithelial cell restitution comprises the migration of epithelial cells from the edge of mucosal injury into the wounds within hours to maintain mucosal integrity and homeostasis in the gut. In our experiments, epithelial cell migraton was abolished by neutralizing antibodies against TGF-β, indicating a TGF-β-dependent effect. TGF-β is the key mediator within an orchestra of growth factors and cytokines inducing epithelial cell restitution [19, 22–25]. We already showed that SP modulates epithelial cell restitution in vitro mediated by fibroblasts while CGRP had no effect in this experimental setting [14]. Using mast cells, a modulatory effect on epithelial cell proliferation and migration was demonstrated. Mast cells represent an interesting target because they play a major role in modulating inflammatory responses in several tissues and they are located in close proximity to nervous fibres throughout the gut. Moreover, it has been reported that they are present in surgical resections of patients with ulcerative colitis and Crohn`s disease (CD). While it could be shown that in healthy controls mast cells were localised in the gut mucosa, their amount increased significantly in the submucosa and the muscularis propria in CD patients, indicating a disease-depending effect [26].
We showed earlier that stimulation of primary afferents in the colonic mucosa of rats with the neurotoxin capsaicin induced a significant immigration of inflammatory cells, an increase of bromodeoxyuridine (BrdU) uptake into the surface epithelium and an increase of DNA content of colonic mucosa [12]. More recently, we have shown that SP induced epithelial cell migration via stimulation of fibroblasts [14]. We did not detect any direct effects of SP or CGRP on epithelial cell function in unconditioned IEC-18 or CaCo-2 cells. CaCo-2 cells express NK-1 receptors after exposure to pro-inflammatory cytokines while unexposed CaCo-2 cells do not express the receptor [13].
Currently, we cannot exclude for sure the possibility that the supernatants of activated mast cells induce the expression of CGRP receptors on epithelial cells. This could lead to an interaction of a remaining low dose of CGRP in the supernatants with CGRP receptors on epithelial cells and thus to the observed effects in this study. This would, however, imply the unlikely possibility that CGRP from mast cell supernatants, diluted 1:160, is stable and bioactive in the cell culture media for more than 24 h.
Therefore, the in vivo observed BrdU uptake into epithelial cells indicating an increased nucleotide turnover and presumably epithelial cell proliferation seems more likely related to actions of the neurotransmitters SP and CGRP mediated via fibroblasts or mast cells.
There are some remarkable differences between the cells used in these experiments such as species differences, differences between non-transformed and cancer-derived cell lines and small-intestine-derived vs. colon-derived epithelial cells. The murine-derived mast cells certainly imply other abilities and receptors as human intestinal mast cells but, as these are not available to date, our experiments have to remain the best possible approach. However, sufficient data are published to show that colonic and intestinal epithelial cells do not differ in the general mechanisms of epithelial cell restitution like epithelial cell migration, proliferation and differentiation [25]. Furthermore, molecular properties of SP, CGRP and TGF-β are similar in these species.
In summary, CGRP and extrinsic primary afferent neurons appear to play an important role in mucosal protection and repair. In addition to its known effects on mucosal blood flow, we were able to show further effects of CGRP on epithelial cell restitution mediated by mast cells. Both the increase in mucosal blood flow and the induction of epithelial cell restitution are events that occur early after mucosal injury. The release of CGRP from neuroeffector junctions of extrinsic afferents was detected as early as 4 h after the induction of mucosal injury giving an ideal time frame to develop its protective properties [9]. This observation underlines an important role for the interaction of mast cells, epithelial cell function and primary extrinsic afferent neurons. While this may lead to a better understanding of the orchestra of mucosal defence and repair, it is still far from reaching therapeutic approaches. Further studies have to verify our results in experiments with selective receptor blockers or specific knock-outs.
References
Evangelista S, Meli A (1989) Influence of capsaicin-sensitive fibres on experimentally-induced colitis in rats. J Pharm Pharmacol 41(8):574–575
Reinshagen M, Patel A, Sottili M, French S, Sternini C, Eysselein VE (1996) Action of sensory neurons in an experimental at colitis model of injury and repair. Am J Physiol 270(1 Pt 1):G79–G86
Reinshagen M, Rohm H, Steinkamp M, Lieb K, Geerling I, Von Herbay A, Flamig G, Eysselein VE, Adler G (2000) Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology 119(2):368–376
Holzer P (2006) Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125:70–75
Holzer P (1989) Peppers, capsaicin, and the gastric mucosa. JAMA 261(22):3244–3245
Holzer P (1988) Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24(3):739–768
Endoh K, Baker M, Leung FW (1991) Mechanism of intragastric nicotine protection against ethanol-induced gastric injury. Dig Dis Sci 36(1):39–46
Holzer P (1991) Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43(2):143–201
Eysselein VE, Reinshagen M, Cominelli F, Sternini C, Davis W, Patel A, Nast CC, Bernstein D, Anderson K, Khan H et al (1991) Calcitonin gene-related peptide and substance P decrease in the rabbit colon during colitis. A time study. Gastroenterology 101(5):1211–1219
Kawasaki H, Takasaki K, Saito A, Goto K (1988) Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 335(6186):164–167
Raybould HE, Sternini C, Eysselein VE, Yoneda M, Holzer P (1992) Selective ablation of spinal afferent neurons containing CGRP attenuates gastric hyperemic response to acid. Peptides 13(2):249–254
Hoffmann P, Mazurkiewicz J, Holtmann G, Gerken G, Eysselein VE, Goebell H (2002) Capsaicin-sensitive nerve fibres induce epithelial cell proliferation, inflammatory cell immigration and transforming growth factor-alpha expression in the rat colonic mucosa in vivo. Scand J Gastroenterol 37(4):414–422
Goode T, O’Connor T, Hopkins A, Moriarty D, O’Sullivan GC, Collins JK, O’Donoghue D, Baird AW, O’Connell J, Shanahan F (2003) Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by proinflammatory cytokines and mediates proliferation in response to substance P. J Cell Physiol 197(1):30–41
Felderbauer P, Bulut K, Hoeck K, Deters S, Schmidt WE, Hoffman P (2007) Substance P induces intestinal wound healing via fibroblasts—evidence for a TGF-beta-dependent effect. Int J Colorectal Dis 22(12):1475–1480
He SH (2004) Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol 10(3):309–318
Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME (1989) Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med 170(1):245–257
Wodnar-Filipowicz A, Heusser CH, Moroni C (1989) Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature 339(6220):150–152
Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE (1989) Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature 339(6219):64–67
Dignass AU, Podolsky DK (1993) Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology 105(5):1323–1332
Hoffmann P, Dignass AU, Zeeh JM, Mazurkiewicz J, Holtmann G, Gerken G (2001) Epithelial cell-derived components induce transforming growth factor-alpha mRNA expression and epithelial cell proliferation in vitro. Eur J Gastroenterol Hepatol 13(11):1333–1340
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Ciacci C, Lind SE, Podolsky DK (1993) Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology 105(1):93–101
Dignass AU, Podolsky DK (1996) Interleukin 2 modulates intestinal epithelial cell function in vitro. Exp Cell Res 225(2):422–429
Dignass AU, Stow JL, Babyatsky MW (1996) Acute epithelial injury in the rat small intestine in vivo is associated with expanded expression of transforming growth factor alpha and beta. Gut 38(5):687–693
Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK (2003) Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol 162(2):597–608
Andoh A, Deguchi Y, Inatomi O, Yagi Y, Bamba S, Tsujikawa T, Fujiyama Y (2006) Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol Rep 16(1):103–107
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerem Bulut and Peter Felderbauer contributed equally.
Rights and permissions
About this article
Cite this article
Bulut, K., Felderbauer, P., Deters, S. et al. Sensory neuropeptides and epithelial cell restitution: the relevance of SP- and CGRP-stimulated mast cells. Int J Colorectal Dis 23, 535–541 (2008). https://doi.org/10.1007/s00384-008-0447-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0447-7