Abstract
Background and aims
Colorectal cancer (CRC) is the third most common cause of cancer-related death in Taiwan. During the past 20 years, several advances have improved the treatment outcome and quality of life of CRC patients. The purpose of this study was to identify the changes in the clinicopathological features and outcome of CRC over this period.
Materials and methods
Based on the computerized database of the Taipei Veterans General Hospital, between January 1981 and December 2000, 5,474 CRC patients were identified and divided into 2 groups based on the date of treatment (1981–1990 and 1991–2000). The clinicopathological features, outcome, and prognostic factors were analyzed and compared.
Results/findings
The age at onset of cancer was 61 years in the 1980s group and 66 years in the 1990s group. The frequency of rectal tumors decreased from 50% in the 1980s group to 44% in the 1990s group. Tumor, nodes, metastasis (TNM) stage distribution, surgical mortality, and anastomosis leakage were similar in the two groups. However, the 5-year overall survival rate was better in the 1990s group (56%) than that in the 1980s group (50%, P = 0.001). For rectal cancer patients, the local recurrence rate was lower in the 1990s group (6%) than that in the 1980s group (10%, P < 0.01). In stage III CRC, the 5-year overall survival rate was significantly higher in the 1990s group (54%) than that in the 1980s group (48%, P = 0.011). TNM stage was the most important independent prognostic factor for overall and disease-free survivals, followed by differentiation grade, CEA level, and treatment period.
Interpretation/conclusion
Advances in surgical technique and more standard use of chemotherapy have improved CRC outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most frequent cause of cancer deaths worldwide [1]. In Taiwan, the incidence of CRC has increased markedly in the past 30 years. It was 6/100,000 in 1970 and increased to 31/100,000 in 2000. There were 7,213 new cases of CRC resulting to 3,376 CRC-related deaths in 2000 [2].
Over the past 30 years, several events have occurred that have influenced the treatment outcome and quality of life of CRC patients [1, 3]. More standard use of 5-FU-based chemotherapy has decreased tumor recurrence in stage III patients. Before the introduction of chemotherapy, 67% of stage III CRC patients developed tumor recurrence within 5 years; with chemotherapy this decreased to 55% [4, 5]. In patients with rectal cancer, total mesorectal excision (TME) and preoperative chemoradiotherapy (CCRT) have improved local tumor control. In patients who received TME and CCRT, the recurrence rate was lower than 10%, which was significantly lower than the 33% recurrence rate in patients who did not receive TME and CCRT [6, 7]. Improvements in surgical technique have increased the possibility of sphincter-saving operations, thus improving patients’ quality of life by avoiding the need for a permanent colostomy [8–10].
The purpose of this study was to review the changes in the clinicopathological features and to investigate the long-term outcomes of CRC treated in a tertiary referring medical center during the past 26 years.
Materials and methods
From January 1981 to December 2000, a total of 5,577 patients with colorectal cancer had surgery in the Taipei Veterans General Hospital. The clinical data after 1995 were prospectively stored in a computer database while data before 1995 were prospectively stored in a punch card and then converted to computer storage. The data included: (1) name, gender, age, family history of cancers, major medical problems, tumor markers including carcinoembryonic antigen (CEA) and cancer antigen (CA 19-9) levels; (2) location, gross appearance, tumor, nodes, metastasis (TNM) stage, differentiation, and the important pathologic prognostic features of the tumor; and (3) types of operations, complications, recurrences, and follow-up status.
The location of the tumor includes the cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and rectum. Right colon means location proximal to (including) the transverse colon, while left colon means location from the splenic flexure (included) to the sigmoid colon. Rectal tumor means tumors located below 15 cm from the anal verge and are classified as upper (11–15 cm), middle (6–10 cm) and lower (≤5 cm from the anal verge). Tumors in the colon were operated with a wide excision of the mesentery and associated lymphovascular tissue extending one vessel proximal and distal to the main feeding vessels. High ligation of inferior mesenteric vessels has been the standard procedure in the resection of sigmoid and rectal cancers. For the mobilization of the rectum, sharp dissection with preservation of the rectal fascia propria has been always followed. However, lateral lymph node dissection is not a routine procedure. Since 1990, two important changes have been occurred in our hospital. The first was complete mobilization of the rectum down to the pelvic floor with transaction of the rectum at the anorectal junction has been practiced for the middle and lower rectal cancers. The second was a more aggressive attitude of chemotherapy for the stage III and stage IV cancers. We therefore chose 1990 as a dividing point to analyze the data. When the tumor was completely removed without gross or microscopic residual tumor or metastatic lesions, the operation was classified as curative otherwise it was classified as palliative.
Before discharge from the hospital, the patients were informed about the follow-up protocol, which included regular visits every 3 months for the first 2 years, every 6 months for the subsequent 3 years, and at least every year thereafter. Routine follow-up examinations including a precise physical examination, rectodigital examination, CEA levels, chest X-rays, abdominal sonograms, and/or abdominal computerized tomography (CT) scan. If there was any suspicion of tumor recurrence, further study such as chest CT scan, whole body bone scan, and even whole body positron emission tomography (PET) scan (available in our hospital since 1996) were done to identify the site of recurrence. The definitions of local recurrence of the rectum included recurrence over or around the anastomosis, in the rectal fossa or pelvic cavity, which was proved by pathological confirmation or progressively increasing size in radio-image study. All pathological reports were reviewed and the classification of the TNM system was revised according to the fifth edition of the AJCC cancer staging manual [11].
Statistical analysis
The endpoint measurements used in this study were the percentages of overall survival from the date of surgery. The distribution of each clinicopathological variable was compared using the two-tailed Fisher’s exact procedure and the chi-square test. The numerical values were compared using Student’s t test. Data are expressed as the mean ± standard deviation (SD). Kaplan–Meier survival curves were compared using the log rank test. Statistical significance was defined as P < 0.05. Multivariate analysis was carried out using the Cox proportional hazards model. Variables with P < 0.1 in the univariate analysis were entered into the multivariate analysis (SPSS for Windows version 10.0).
Results
A total of 5,577 patients were enrolled in our database, but 103 patients were excluded due to insufficient or incorrect data. There were 303 (5.5%) patients who had unresectable tumors; they either had no surgical resection of the tumor or only received diversion or bypass surgery. Based on the date of treatment, the patients were divided into two groups (1981 to 1990 and 1991 to 2000). As shown in Table 1, the age at onset of cancer was 61 years in the 1980s group and 66 years in the 1990s group. The frequency of rectal tumors decreased from 50% in the 1980s group to 44% in the 1990s group. There were more poorly differentiated tumors in the 1980s group. The distribution of TNM stage was similar in the two groups. In younger patients (<50 years of age), the frequency of proximal colon tumors (proximal to the splenic flexure) was similar to that in older patients. In the 1990s, the harvested lymph nodes were significantly more than that in the 1980s. This may be caused by a more aggressive attitude of surgical resection or more detailed examination of the surgical specimen. Of the rectal cancer patients, 37% (383/1,040) received an exstirpation (abdominoperineal resection or Hartmann operation) and 63% (657/1,040) received anterior resection with reconstruction in the 1980s group compared to 21% (294/1,368) and 79% (1,074/1,368) in the 1990s group (P < 0.001). As shown in Tables 2 and 3, the clinicopathological features were similar when colon and rectal cancers were analyzed separately.
The surgical mortality and the rate of anastomosis leakage were similar in the 1980s and 1990s groups. As shown in Table 4, the 5-year overall survival rate was better in the 1990s group (56%) than that in the 1980s group (50%). In patients with stage I CRC, there was no significant difference in the 5-year (1980s, 79%; 1990s, 85%) and 10-year (1980s, 68%; 1990s, 71%) survival rates between the 2 groups. In patients with stage III CRC, the 5-year survival rate was significantly better in the 1990s group (54%) than that in the 1980s group (48%). In patients with stage IV CRC, the 1-year and 3-year survival rates (1-year, 51%; 3-year, 14%) in the 1990s group were also better than that in the 1980s group (1-year, 39%; 3-year, 11%). However, when analyzed separately, the better survival of cases in the 1990s group were not significant in stage III and IV rectal cancers as shown in Tables 5 and 6. In the rectal cancers, the local recurrence rate was 6% in the 1990s group, which was better than that in the 1980s group (10%).
On univariate analysis, the clinicopathological factors affecting the overall survival of the CRC were TNM stage, differentiation grade, high CEA level, gender, and treatment period (1980s vs 1990s) (Table 7). In the multivariate analysis, the most important factor was TNM stage, followed by high CEA level and treatment period (Table 8). TNM stage, differentiation grade, high CEA level, and treatment period were independent prognostic factors for CRC disease-free survival.
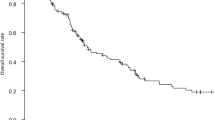
In the 1980s group, 50% of stage III patients and 38% of stage IV patients received chemotherapy. In the 1990s group, the chemotherapy rates increased to 64% of stage III patients and 55% of stage IV patients. The patients in the 1990s who received chemotherapy (either in stage III or in stage IV tumors) were significantly more than those in the 1980s. However, there appeared to be no significant benefit of chemotherapy in the 1980s group. In stage III patients, the 5-year overall survival rate was 45% with chemotherapy and 48% without chemotherapy (Fig. 1), while in stage IV patients, the 1-year overall survival rate was 45% with chemotherapy and 42% without chemotherapy. However in the 1990s group, the benefit of chemotherapy was significant. In stage III patients, the 5-year overall survival rate was 57% with chemotherapy and 45% without chemotherapy, while in stage IV patients, the 1-year overall survival rate was 65% with chemotherapy and only 32% without chemotherapy.
Discussion
Our series consisted of CRC cases diagnosed from 1981 to 2000. The number of cases per year increased progressively and was comparable to the trend of the incidence of CRC in Taiwan [2]. In the 1990s group, the mean age at onset of colorectal cancer was 66 years, which was older than that in the 1980s group (61 years). The annual report of the Taiwan cancer registry showed that the mean age at CRC diagnosis was 64 years in 1995 and 67 years in 2000 [2]. It is interesting to note that our series showed a significant decrease of the tumor in the rectum. The frequency of rectal tumors was 50% in the 1980s group, but this decreased to 44% in the 1990s group (P < 0.001). Several epidemiologic reports have shown a similar trend [12–14]. Several studies have indicated that mass screening with sigmoidoscopy and possible polypectomy could decrease the incidence of rectal cancer [15–17].
The decreased rate of exstirpation from 37% in the 1980s to 21% in the 1990s reflected the trend of aggressive preservation of the anus based on better realization of distal cancer spread and improvement of surgical technique [18, 19]. The low leakage rate in colon cancer operation was not particular. However, we were surprised by the low leakage rate of rectal anastomosis. It might be caused by several reasons. Only clinical leakage was recorded and analyzed, which might underestimate the subclinical leakage. Furthermore, the high anastomosis of the resection of upper rectal cancer was included for analysis, which might dilute the leakage rate of the low anastomosis of the resection of middle and low rectal cancers. The leakage rate of the resection of rectal cancer below 10 cm from the anal verge was 1.6% and 3.4% in the 1980s and 1990s, respectively. The significant increase of leakage rate in the 1990s (1990s, 2.9%; 1980s, 1.3%) might be caused by increased cases of low anastomosis (1990s, 71%; 1980s, 63%).
Several clinicopathological factors including TNM stage, differentiation grade, gender, CEA level, and the treatment period affected the overall survival. However, analysis of our data showed that gender had no significant impact on disease-free survival. The different influences of the female gender between overall and disease-free survival may be caused by the longer female life expectancy (female, 78 years vs male, 71 years in Taiwan) [2].
Our results showed that there was no change in the distribution of proximal tumor in the young age group. There was no difference of the percentage of the proximal colon between the young and old age group. In contrast, other studies have reported that younger CRC patients were more likely to have proximal colon tumors [20–22].
Advances in surgical technique and chemotherapy have improved the outcome of CRC patients [23–26]. High ligation of the inferior mesenteric vessels, sharp dissection to mobilize the rectum with removal of most mesorectal tissue have been standard procedures used in the radical resection of rectal cancer in our hospital for a long time. However, since 1990, a complete mobilization of the rectum down to the pelvic floor, resection of the rectum near the anorectal junction for the middle and lower rectal tumors have been used to resect the rectal tumors. This procedure fulfills the technique of total mesorectal excision (TME). The significant improvement of stage II and the significant decrease of the local recurrence rate of all rectal cancer may be related to this change of surgical technique. The effect of neoadjuvant chemoradiotherapy (CCRT) couldn’t be evaluated because the standard regimen of CCRT has been introduced to our hospital since 2000.
To correctly assess the lymph node status of the tumor is mandatory for accurate staging. Inadequate resection and examination of the lymph node could downstage the tumor and preclude the patient from necessary adjuvant therapy. The agreement in determining a universal valid minimum number of lymph nodes to establish an accurate stage has not yet been reached [27, 28]. The College of American Pathologists consensus meeting publications recommended that at least 12 lymph nodes were sufficient for accurate staging [3]. In our previous study, a shift of N2 to N1 was noted when the number of harvested lymph nodes was below 10 [29]. Although the number of harvested lymph nodes didn’t influence the survival outcome in this study, the harvested number in 1990s was significantly increased, which reflected the more aggressive attitude of surgery and more detailed pathological examination.
Since the introduction of standardized 5-FU-based chemotherapies, including the Mayo and de Gramont regimens, the outcome of stage III and IV CRC patients have significantly improved [30–33]. Our series showed similar results. The 5-year overall survival rate of stage III patients was better in the 1990s (54%) than that in the 1980s (48%). In the 1990s, the 5-year overall survival rate of stage III CRC patients receiving chemotherapy was 57% with an approximately 20% decrease in tumor recurrence compared to stage III patients not receiving chemotherapy (45%). It is interesting to note that this improvement of survival with chemotherapy could not be observed in the 1980s group. Before 1990, the effect of adjuvant chemotherapy was controversial. The policy and regimen of adjuvant chemotherapy in our hospital were not standardized. Which patients should receive chemotherapy were dependent on the subjective judgement of the surgeon. Many patients did not receive the complete course of chemotherapy. All of these factors may influence the result of chemotherapy. Although stage III patients who received chemotherapy increased from 50% in the 1980s group to 67% in the 1990s group, this rate was relative low compared to other series [34–37]. The factors that may have contributed to the low chemotherapy rate in advanced cancer patients include transportation difficulties in getting to the hospital and the old age of the patients. In the 1990s group, of the 337 stage III disease patients who did not receive chemotherapy, 117 cases (35%) had transportation difficulties and 186 cases (55%) were old age with poor general condition. Several studies have found that chemotherapy has the same benefit in younger and older patients, but older patients are less frequently treated [38, 39]. Jessup et al. have also noted that patients receiving adjuvant therapy for stage III colon cancer, especially low-grade cancer, had an increased survival benefit [40].
Recent advances in understanding tumor biology have led to the development of novel drugs that target different pathways, which are important in malignant phenotypes [41–44]. Thus, in the future, individualized treatment based on genetic tumor profiles may become possible. The current challenge is to identify the risk factors, which may predict the recurrence of CRC and increase the rate of treatment in patients who may respond to chemotherapy.
Conclusion
The results of the treatment of CRC have improved during the past 20 years. More accurate staging, improved surgical technique in rectal surgery, and more aggressive attitude of chemotherapy were the most important contributive factors.
References
Weitz J, Koch M, Debus J et al (2005) Colorectal cancer. Lancet 365:153–165
Health and Vital Statistics (2000) Department of Health, ROC, Taipei
Compton CC, Fielding LP, Burgart LJ et al (2000) Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124:979–994
Gill S, Loprinzi CL, Sargent DJ et al (2004) Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22:1797–1806
Marsoni S, International Multicenter Pooled Analysis of Colon Cancer Trials Investigators (2001) Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. International Multicenter Pooled Analysis of Colon Cancer Trials Investigators. Semin Oncol 28:14–19
Dahlberg M, Pahlman L, Bergstrom R et al (1998) Improved survival in patients with rectal cancer: a population-based register study. Br J Surg 85:515–520
Enker WE, Thaler HT, Cranor ML et al (1995) Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181:335–346
Zhao GP, Zhou ZG, Lei WZ et al (2005) Pathological study of distal mesorectal cancer spread to determine a proper distal resection margin. World J Gastroenterol 11:319–322
Rullier E, Laurent C, Bretagnol F et al (2005) Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 241:465–469
Schiessel R, Novi G, Holzer B et al (2005) Technique and long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum 48:1858–1865 (discussion 1865–1867)
American Joint Committee on Cancer (1997) AJCC cancer staging manual, 5th edn. Springer, Berlin Heidelberg New York
Mostafa G, Matthews BD, Norton HJ et al (2004) Influence of demographics on colorectal cancer. Am Surg 70:259–264
Mensink PB, Kolkman JJ, Van Baarlen J et al (2002) Change in anatomic distribution and incidence of colorectal carcinoma over a period of 15 years: clinical considerations. Dis Colon Rectum 45:1393–1396
Takada H, Ohsawa T, Iwamoto S et al (2002) Changing site distribution of colorectal cancer in Japan. Dis Colon Rectum 45:1249–1254
Newcomb PA, Norfleet RG, Storer BE et al (1992) Screening sigmoidoscopy and colorectal cancer mortality. J Natl Cancer Inst 84:1572–1575
Selby JV, Friedman GD, Quesenberry CP Jr et al (1992) A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 326:653–657
Ransohoff DF, Lang CA (1993) Sigmoidoscopic screening in the 1990s. JAMA 269:1278–1281
Williams NS, Dixon MF, Johnston D (1983) Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients’ survival. Br J Surg 70:150–154
Kameda K, Furusawa M, Mori M et al (1990) Proposed distal margin for resection of rectal cancer. Jpn J Cancer Res 81:100–104
Simstein NL, Kovalcik PJ, Cross GH (1978) Colorectal carcinoma in patients less than 40 years old. Dis Colon Rectum 21:169–171
Qing SH, Rao KY, Jiang HY et al (2003) Racial differences in the anatomical distribution of colorectal cancer: a study of differences between American and Chinese patients. World J Gastroenterol 9:721–725
Marble K, Banerjee S, Greenwald L (1992) Colorectal carcinoma in young patients. J Surg Oncol 51:179–182
Faivre-Finn C, Bouvier-Benhamiche AM, Phelip JM et al (2002) Colon cancer in France: evidence for improvement in management and survival. Gut 51:60–64
Finn-Faivre C, Maurel J, Benhamiche AM et al (1999) Evidence of improving survival of patients with rectal cancer in France: a population based study. Gut 44:377–381
Porter GA, Soskolne CL, Yakimets WW et al (1998) Surgeon-related factors and outcome in rectal cancer. Ann Surg 227:157–167
Smith JA, King PM, Lane RH et al (2003) Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 90:583–592
Scott KW, Grace RH (1989) Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg 76:1165–1167
Maurel J, Launoy G, Grosclaude P et al (1998) Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer 82:1482–1486
Shen M-Y, Lin J-K, Lin T-C et al (2003) Prognostic significance of lymph node metastasis in resected colorectal cancer. J Soc Colon Rectal Surgeon (Taiwan) 14:7–14
Buroker TR, O’Connell MJ, Wieand HS et al (1994) Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 12:14–20
Francini G, Petrioli R, Lorenzini L et al (1994) Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 106:899–906
Moertel CG (1994) Chemotherapy for colorectal cancer. N Engl J Med 330:1136–1142
Moertel CG, Fleming TR, Macdonald JS et al (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
Arkenau HT, Bermann A, Rettig K et al (2003) 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol 14:395–399
Sargent DJ, Goldberg RM, Jacobson SD et al (2001) A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345:1091–1097
Potosky AL, Harlan LC, Kaplan RS et al (2002) Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol 20:1192–1202
Ayanian JZ, Zaslavsky AM, Fuchs CS et al (2003) Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol 21:1293–1300
Fata F, Mirza A, Craig G et al (2002) Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: a 10-year experience of the Geisinger Medical Center. Cancer 94:1931–1938
Iwashyna TJ, Lamont EB (2002) Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 20:3992–3998
Jessup JM, Stewart A, Greene FL et al (2005) Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 294:2703–2711
Fernando NH, Hurwitz HI (2003) Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol 30:39–50
O’Dwyer PJ, Benson AB 3rd (2002) Epidermal growth factor receptor-targeted therapy in colorectal cancer. Semin Oncol 29:10–17
Kitisin K, Mishra L (2006) Molecular biology of colorectal cancer: new targets. Semin Oncol 33(Suppl 11):14–23
Wilson RH (2006) Novel therapeutic developments other than EGFR and VEGF inhibition in colorectal cancer. Oncologist 11:1018–1024
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ju, JH., Chang, SC., Wang, HS. et al. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorectal Dis 22, 855–862 (2007). https://doi.org/10.1007/s00384-007-0293-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-007-0293-z