Abstract
Background and aims
Clinical anastomotic leakage remains a major problem after anterior or low anterior resection for rectal cancer. The aim of this study was to assess the association between risk factors and anastomotic leakage and postoperative mortality.
Materials and methods
Two hundred seventy-six elective anterior or low anterior resections with anastomosis were performed and documented on-line from January 1995 to December 2004. Univariate and multivariate analyses with Bonferroni adjustment were carried out to identify relevant risk factors.
Results
The rate of anastomotic leakage was 14.9% (41 of 276 patients) with a mortality of 12.2% (5 of 41 patients). Overall mortality was 2.5% (7 of 276 patients). Multiple regression analysis showed that smokers had an increased risk of anastomotic leakage [odds ratio (OR), 6.42; 95% confidence interval (CI), 2.68–15.36] as well as patients with coronary heart disease (OR, 7.79; 95% CI, 2.52–24.08). Smokers (OR, 13.20; 95% CI, 2.48–7.24) and patients with coronary heart disease (OR, 23.46; 95% CI, 4.33–27.04) also had an increased risk of postoperative mortality in the univariate analysis as well as patients with anastomotic leakage (OR, 16.25; 95% CI, 3.04–16.92).
Conclusions
Smoking and coronary heart disease are important risk factors for anastomotic leakage and postoperative mortality after elective resection for rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical anastomotic leakage, which occurs in 10–16% of cases, is the most feared and serious complication in rectal cancer surgery, as it considerably contributes to postoperative morbidity and mortality [1–6]. Accounting for a third of all complications, leakage at the anastomotic site is not only the decisive mortality factor in surgery of rectal cancer [1, 7] but is also associated with a higher local recurrence rate and lower long-term survival [8–13]. Moreover, long-term functional outcome may be adversely affected by anastomotic leakage [14].
Knowledge of the factors influencing anastomotic healing is thus of decisive importance, especially with regard to the consequences for perioperative management or tactical considerations of surgical procedures. As a great number of risk factors are controversially discussed in the literature, the aim of this prospective observational study was to evaluate the risk factors for anastomotic leakage after elective surgery for rectal cancer in our patient population.
Materials and methods
Study design
All patients who underwent surgery for colorectal cancer were documented on-line in an open prospective observational study. Documentation started in January 1995 and involved a surgical part filled out immediately after the procedure by the surgeon and a postoperative part filled out by the ward physician on the day of discharge. Basic data were recorded as well as detailed information on history, risk factors, preoperative diagnostics, surgical procedure, intraoperative findings, histopathological work-up, and postoperative course.
According to the aim of this investigation, all patients were included in the study, who underwent elective anterior continuity resection for primary rectal cancer between January 1995 and December 2004. Exclusion criteria were emergency interventions, re-operations, secondary tumors, and multivisceral resection. A total of 276 patients were included and analyzed.
Surgical procedure
Preoperative preparation and intervention were standardized. Beside orthograde intestinal lavage with polyethylene glycol, perioperative antibiotic prophylaxis was carried out with aminopenicillin or a cephalosporin in combination with metronidazole and, since the end of 2003, with ampicillin/sulbactam. Anterior rectal resection was done according to oncological principles. After central ligature of the inferior mesenteric artery and radical lymphadenectomy, partial mesorectal excision (PME) was carried out in tumors of the upper third of the rectum and total mesorectal excision (TME) in tumors of the middle and lower third of the rectum. Abdominoperineal rectal excision was done with a tumor location of less than 2 cm from the dentate line. Intersphinctal rectum resection was done in tumors higher than 2 cm from the dentate line if the rectum below the tumor could not be closed by linear stapling. Reconstruction for tumors of the upper third of the rectum was done by sutured or stapled end-to-end descendorectostomy (straight anastomosis). Colon pouch reconstruction was usually carried out in tumors of the middle or lower third. Colopouch-rectal anastomoses were stapled and colopouch-anal anastomoses were sutured. A protective ileostoma was generally created in these deep anastomoses. Pelvic drainages were routinely inserted.
Definition of anastomotic leakage
Only clinically manifest and thus relevant anastomotic leakage was recorded. This was present in cases with fecal secretion via indwelling drainage, surgical wound or vagina, or by CT detection with rectal contrast application. Computed tomography was indicated in patients with clinical deterioration, abnormal abdominal findings, putrid rectal secretion, turbid drainage secretion, or air escaping via the drainage.
Analyzed variables
The variables included in the analysis were patient-, tumor-, and therapy-related parameters. Patient-related variables comprised age, gender, data on cardiovascular risk factors [nicotine abuse (minimum 5 pack years), alcohol abuse (mean of a minimum of 20 g/day over the last 3 months), obesity (body mass index (BMI) >30)], and comorbidity [diabetes mellitus (if medical treatment was established), hypertension, coronary heart disease (CHD, alteration in EKG and/or angina pectoris in anamnesis and/or specific medical treatment), and chronic obstructive pulmonary disease (COPD)]. Tumor-related variables were tumor stage according to the International Union against Cancer (UICC) and tumor height from the dentate line. Therapy-related variables included neoadjuvant therapy, extent of mesorectal excision (PME and TME), type of reconstruction (straight, pouch-rectal, and pouch-anal anastomoses), anastomotic technique (stapler and manual suture) as well as protective ileostomy (Table 1).
Statistical evaluation
The occurrence of anastomotic leakage was correlated with the variables mentioned above (Table 1). Statistical analysis was done using Statistical Package for the Social Sciences for Windows. Data were initially analyzed by univariate χ 2-test. Variables with a p value < 0.1 in the univariate analysis were subsequently subject to multivariate analysis by logistic regression. A Bonferroni adjustment to account for multiple testing was performed by lowering the α level for each test to 0.003 to reach an overall statistical significance level of less than 5% (p < 0.05).
Results
Two hundred seventy-six patients underwent elective anterior rectal resection for primary rectal cancer between January 1995 and December 2004. The median age was 63 years (range, 35–90). Male patients clearly dominated with about 60%. Arterial hypertension was the predominant risk factor in almost one third of the patients, followed by regular alcohol abuse in nearly one fifth, and obesity and nicotine abuse in 15%. Other risk factors were CHD in 11.2%, diabetes mellitus in 9.4%, and COPD in 8%. More than 50% of the patients presented with metastases, 31.9% with lymph node metastases (UICC III), 18.5% with distant metastases (UICC IV). Twenty-six percent of the patients showed advanced tumors without metastases (UICC II) and 23.6% an early tumor stage (UICC I). Rigid rectoscopy was performed to determine the tumor distance from the dentate line. Four groups were differentiated (group I, <4 cm; group II, 4–8 cm; group III, 8–12 cm; and group IV, >12 cm). In about 50%, the aboral tumor height was 8 cm from the dentate line. Less than one fourth of the tumors were situated above 12 cm. All patient and tumor parameters are given in Table 2.
Based on the number of T4 tumors, neoadjuvant radiochemotherapy was carried out in only 6.9% of the patients. The extent of mesorectal excision correlated with the tumor localization. TME (60.5%) was performed in all tumors of the middle and lower third, PME with an aboral safety margin of at least 5 cm only in tumors of the upper third. Colon-pouch reconstruction was carried out if the rectal stump was less than 4 cm. The length of the rectal stump was intraoperatively estimated by the surgeon, who had preoperatively determined the distance of the tumor from the dentate line by rectoscopy. Forty-three patients received a pouch-rectal and 70 a pouch-anal anastomosis. Straight anastomoses were performed in tumors above 4 cm. All perianal anastomoses at the dentate line were done by manual technique. Nearly 90% of all other anastomoses were stapled. The deeper the anastomosis, the higher was the number of created protective stomas. Thus, a protective ileostoma was created in 90% of all anal anastomoses, in 83.7% of pouch-rectal anastomoses, and in less than one third of all straight anastomoses (Table 3).
Clinically relevant anastomotic leakage was seen in 41 patients (14.9%) during primary hospitalization. More than 80% of these patients underwent surgical revision. Five (1.8%) patients died from therapy-refractory septic multiorgan failure despite consequent surgical (programmed lavage) and intensive care management. Other major complications were intra-abdominal abscesses with no evidence of anastomotic leakage (2.5%), intraoperative organ damage (1.8%), postoperative hemorrhage (1.5%), as well as burst abdomen (1.1%). None of the patients died from these complications (Table 4).
The main non-surgical complication was decompensated cardiac insufficiency (NYHA IV) and acute myocardial infarction. Two (0.7%) of the eight patients (2.9%) with this complication died. Other non-surgical complications were pneumonia (2.5%), decompensated renal insufficiency (2.5%), decompensated liver insufficiency (1.1%), apoplectic stroke (0.4%), as well as catheter infections (2.2%; Table 4). Total mortality in this study was thus 2.5%.
Based on the univariate analysis of the variables by the χ 2-test listed in Table 1, nicotine abuse (p < 0.001) and CHD (p < 0.001) were found to be significant risk factors for anastomotic leakage after elective surgery for rectal cancer (Table 5). The multivariate logistic regression analysis also showed a significant association between the two variables and anastomotic leakage (p < 0.001; Table 6). Diabetes mellitus calculated with a p value of 0.067 in the univariate analysis was not significant in the multivariate analysis (p = 0.655).
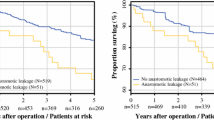
Regarding postoperative mortality, univariate analysis of the variables yielded significant values for nicotine abuse (p < 0.001) and CHD (p < 0.001). Moreover, there was a significant association between anastomotic leakage and postoperative mortality (p < 0.001; Table 7). However, this association was not confirmed in the multivariate analysis (Table 8).
Discussion
Although much progress has been made in the surgery of rectal cancer, about 15% of the patients have clinically manifest anastomotic leakage, which considerably contributes to postoperative morbidity and mortality [1–7]. Anastomotic leakage, moreover, is responsible for an increased rate of local recurrence and thus shorter long-term survival and functional deficits [8–14].
To improve this situation, knowledge of the factors influencing anastomotic healing is of decisive importance. The aim of this prospective observational study was therefore to evaluate risk factors for clinically manifest anastomotic leakage after elective resection for rectal cancer.
The analyzed variables were subdivided into patient-, tumor-, and therapy-related parameters and included most of the variables cited in the literature as potential risk factors for anastomotic leakage. An important aspect in this context is to document the factors that considerably influence comorbidity (nicotine, alcohol abuse, BMI, diabetes mellitus, hypertension, CHD, and COPD).
Until December 2004, neoadjuvant treatment was only administered in T4 cancers and not, as practiced today, in T3 and N+ cancers [15], which explains the apparently small number of patients (6.9%) receiving this treatment.
The 14.9% rate of clinically relevant anastomotic leakage is within the range reported in the literature (8.1 to 15.9%) and is an acceptable value for a teaching clinic [1, 5, 6, 16]. Two of the seven deaths were due to a cardiac event, the other five due to anastomotic leakage. The leakage-related mortality rate was thus 12.2% and total mortality was 2.5%. This is also in agreement with the rate of 0 to 2.3% reported in the literature [1, 4, 7, 16–18].
In this study, nicotine abuse and CHD both were significantly associated with anastomotic leakage after elective surgery for rectal cancer in the univariate and multivariate analysis. Smoking, as a risk factor for anastomotic healing, was also demonstrated by Sorensen et al. [6] in a study on 333 patients. Alberts et al. [19] and Kasperk et al. [20] likewise reported an increased risk in smoking patients. There are thus far no reports in the literature on the other risk factor CHD describing an increased risk for anastomotic healing. However, as CHD is frequently associated with general arteriosclerosis [21], angiopathy may be assumed, which also involves the mesenterial vessels. Thus, it can be presumed that both smoking and CHD are responsible for a disturbed microcirculation [22], which plays a significant role in the increased rate of anastomotic leakage [23]. The risk factor, diabetes mellitus, which missed the significance level with 0.067 in the univariate analysis, may also contribute to this pathomechanism. Vignali et al. [24] reported a correlation between diabetes mellitus and anastomotic leakage. We found no association with alcohol abuse and obesity, which are described by some authors as risk factors for anastomotic healing [1, 6]. There was likewise no relationship between COPD or hypertension and anastomotic leakage or any reports in the literature. Comorbidity, which increases with age, leads to increased postoperative morbidity [25]. However, in most studies, the rate of anastomotic leakage does not increase with age [5, 6, 25, 26]. Our study likewise showed no increase in the anastomotic leakage rate of over 80-year-old patients after radical resection [27]. In contrast to other studies, the male gender was not identified as a risk factor in our cohort [1, 3, 4, 16]. We did not detect a gender-specific difference, which was in agreement with some other studies [5, 26, 28]. Tumor stage had no influence on the rate of anastomotic leakage, neither in this study nor in others [9, 28]. Neoadjuvant therapy, which was rarely carried out in our study, was not a risk factor in this study but was associated with a higher rate of anastomotic leakage in other investigations [4, 19].
A great number of studies describe the height of the anastomosis as a risk factor for the development of leakage: the lower the anastomosis, the higher the risk [1, 4, 16, 19, 24, 29]. However, a detailed description of how accurately the height of anastomosis was determined is mostly missing. Although intraoperative rectoscopic determination is precise, it may endanger fresh anastomoses and was therefore not carried out in this study. However, the height of anastomosis can be deduced from the type of reconstruction, and three groups can thus be differentiated. The anastomosis is at the level of the dentate line in pouch-anal reconstruction. Pouch-rectal reconstruction is carried out if the rectal stump estimated by the surgeon was up to 4 cm long. All straight anastomoses are therefore higher than 4 cm from the dentate line (Table 1). All pouch-anal anastomoses were hand-sutured, whereas most of the higher situated anastomoses were stapled. As other studies, we did not find a correlation between anastomotic height and insufficiency rate [5, 20]. The insufficiency rate of higher anastomoses (16%) was comparable to that of anal anastomoses (15.7%). Thus, it must be concluded that there are other important factors beside height of the anastomosis, as for example, the type of reconstruction [19, 30]. It is possible that a height-related difference may be masked by the type of reconstruction, as straight anastomoses are associated with a higher insufficiency rate than side-to-end anastomoses [31]. The question as to which technique is safer, hand-sewn or stapled anastomosis, is difficult to answer with this study, as the technique is largely dependent on the type of reconstruction. Like other authors, we failed to detect any difference [19, 32–35].
The necessity and significance of an ileostoma to protect the anastomosis is controversially discussed. The protective stoma had no influence on the leakage rate in our study irrespective of the type of reconstruction. Other study groups reported similar results [4, 36]. Whether the consequences of leakage are attenuated by a stoma was not explicitly examined in our study [19, 20]. Our rate of relaparotomies required after anastomotic leakage was quite high (80,5%). Other authors, however, feel that a protective stoma also reduces the rate of leakage [3, 5, 37, 38]. It is known from earlier studies that about one third of all rectal anastomoses may display radiological signs of leakage [39, 40]. Graffner et al. were able to show that a stoma can prevent the clinical manifestation of a small leak. In their randomized study on 50 patients, half of them received a protective stoma. Leakage was detected in 30% of cases via contrast application in both groups. In the group without a stoma, 12% developed clinically relevant anastomotic leakage compared to only 4% in the group with a stoma [40]. This can be explained by the fact that an initially small, silent leak is widened by the distending stool passage and becomes clinically manifest through consecutively drained stool. A diversion stoma may not prevent the rate of radiologically detectable anastomotic leaks but reduces their clinical manifestation and thus postoperative morbidity [41].
Postoperative mortality after elective resection for rectal cancer was significantly associated with nicotine abuse and CHD in the univariate analysis. Furthermore, there was a significant correlation between anastomotic leakage and postoperative mortality. There are thus far no reports in the literature that identify CHD, nicotine abuse, and anastomotic leakage as risk factors for postoperative mortality after rectal resection. The analyses in most studies almost exclusively address the role of variables in anastomotic leakage but not explicitly in postoperative mortality. Age, which different studies consider to be a risk factor for postoperative mortality [18, 42, 43], was not an influencing factor in our study or others [4, 5, 16]. As comorbidity increases with age, comorbidity and not age itself is more likely to be responsible for increased mortality in those studies [25].
Conclusion
CHD and nicotine abuse are significant risk factors for anastomotic leakage after elective surgery for rectal cancer in both the univariate and multivariate analysis. Moreover, these two variables beside anastomotic leakage are associated with postoperative mortality in the univariate analysis. Patients with this risk profile should thus be submitted to the best possible preoperative conditioning, and a protective stoma should be considered in continuity resections. Intensive postoperative monitoring is also strongly recommended. As these patients have an increased mortality risk, immediate and consequent surgical management is mandatory in patients with a suspected leakage.
References
Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M (1998) Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 85:355–358
Averbach AM, Chang D, Koslowe P, Sugarbaker PH (1996) Anastomotic leak after double-stapled low colorectal resection. Dis Colon Rectum 39:780–787
Law WI, Chu KW, Ho JW, Chan CW (2000) Risk factors for anastomotic leakage after low anterior resection with total mesorectal excision. Am J Surg 179:92–96
Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R (2004) Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 6:462–469
Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92:211–216
Sorensen LT, Jorgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jorgensen P (1999) Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg 86:927–931
Agnifili A, Schietroma M, Carloni A, Mattucci S, Caterino G, Lygidakis NJ, Carlei F (2004) The value of omentoplasty in protecting colorectal anastomosis from leakage. A prospective randomized study in 126 patients. Hepatogastroenterology 51:1694–1697
Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240:255–259
Akyol AM, McGregor JR, Galloway DJ, Murray GD, George WD (1991) Anastomotic leaks in colorectal cancer surgery: a risk factor for recurrence? Int J Colorectal Dis 6:179–183
Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, Bokey EL (2003) Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 90:1261–1266
Fujita S, Teramoto T, Watanabe M, Kodaira S, Kitajima M (1993) Anastomotic leakage after colorectal cancer surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol 23:299–302
Petersen S, Freitag M, Hellmich G, Ludwig K (1998) Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. Int J Colorectal Dis 13:160–163
Branagan G, Finnis D (2005) Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 48:1021–1026
Hallbook O, Sjodahl R (1996) Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg 83:60–62
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Luna-Perez P, Rodriguez-Ramirez SE, Gutierrez de la Barrera M, Labastida S (2002) [Multivariate analysis of risk factors associated with dehiscence of colorectal anastomosis after anterior or lower anterior resection for sigmoid or rectal cancer]. Rev Invest Clin 54:501–508
Poon RT, Law WL, Chu KW, Wong J (1998) Emergency resection and primary anastomosis for left-sided obstructing colorectal carcinoma in the elderly. Br J Surg 85:1539–1542
Fazio VW, Tekkis PP, Remzi F, Lavery IC (2004) Assessment of operative risk in colorectal cancer surgery: the Cleveland Clinic Foundation colorectal cancer model. Dis Colon Rectum 47:2015–2024
Alberts JC, Parvaiz A, Moran BJ (2003) Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal Dis 5:478–482
Kasperk R, Philipps B, Vahrmeyer M, Willis S, Schumpelick V (2000) [Risk factors for anastomosis dehiscence after very deep colorectal and coloanal anastomosis]. Chirurg 71:1365–1369
Galloway JM (2002) The epidemiology of atherosclerosis and its risk factors among native Americans. Curr Diab Rep 2:274–281
Lehr HA (2000) Microcirculatory dysfunction induced by cigarette smoking. Microcirculation 7:367–384
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR (1997) Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg 185:105–113
Anonymous (2000) Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 356:968–974
Eckmann C, Kujath P, Schiedeck TH, Shekarriz H, Bruch HP (2004) Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis 19:128–133
Kruschewski M, Germer CT, Rieger H, Buhr HJ (2002) [Radical resection of colorectal carcinoma in the oldest old]. Chirurg 73:241–244
Tonus C, Keller O, Kropp R, Nier H (1996) [Colorectal carcinoma. Which factors are decisive for development of postoperative complications?]. Langenbecks Arch Surg 381:251–257
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240:260–268
Hallbook O, Pahlman L, Krog M, Wexner SD, Sjodahl R (1996) Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg 224:58–65
Hallbook O, Johansson K, Sjodahl R (1996) Laser Doppler blood flow measurement in rectal resection for carcinoma-comparison between the straight and colonic J pouch reconstruction. Br J Surg 83:389–392
Lustosa SA, Matos D, Atallah AN, Castro AA (2001) Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev 3:CD003144
Everett WG, Friend PJ, Forty J (1986) Comparison of stapling and hand-suture for left-sided large bowel anastomosis. Br J Surg 73:345–348
Docherty JG, McGregor JR, Akyol AM, Murray GD, Galloway DJ (1995) Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg 221:176–184
Laurent A, Parc Y, McNamara D, Parc R, Tiret E (2005) Colonic J-pouch-anal anastomosis for rectal cancer: a prospective, randomized study comparing handsewn vs. stapled anastomosis. Dis Colon Rectum 48:729–734
Wong NY, Eu KW (2005) A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum 48:2076–2079
Poon RT, Chu KW, Ho JW, Chan CW, Law WL, Wong J (1999) Prospective evaluation of selective defunctioning stoma for low anterior resection with total mesorectal excision. World J Surg 23:463–467 (discussion 467–468)
Dehni N, Schlegel RD, Cunningham C, Guiguet M, Tiret E, Parc R (1998) Influence of a defunctioning stoma on leakage rates after low colorectal anastomosis and colonic J pouch-anal anastomosis. Br J Surg 85:1114–1117
Ohman U, Svenberg T (1983) EEA stapler for mid-rectum carcinoma. Review of recent literature and own initial experience. Dis Colon Rectum 26:775–784
Graffner H, Fredlund P, Olsson SA, Oscarson J, Petersson BG (1983) Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum 26:87–90
Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H (2005) Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg 92:1137–1142
Killingback M, Barron P, Dent O (2002) Elective resection and anastomosis for colorectal cancer: a prospective audit of mortality and morbidity 1976-1998. ANZ J Surg 72:689–698
Damhuis RA, Wereldsma JC, Wiggers T (1996) The influence of age on resection rates and postoperative mortality in 6457 patients with colorectal cancer. Int J Colorectal Dis 11:45–48
Acknowledgements
We thank Priv.-Doz. Dr. med. Dr. rer. nat. Werner Hopfenmüller, Institute of Medical Informatics, Biometry and Epidemiology, Charité-Medical School, Campus Benjamin Franklin, for his support in working up the statistical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kruschewski, M., Rieger, H., Pohlen, U. et al. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis 22, 919–927 (2007). https://doi.org/10.1007/s00384-006-0260-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-006-0260-0