Abstract
Background
Little is known about the synchronous occurrence of gastrointestinal stromal tumors (GISTs) and other gastrointestinal tumors. We present two cases of an invasive colon cancer with a synchronous small-bowel GIST; immunohistochemistry studies were performed to evaluate possible genetic similarities.
Methods
This paper reports two cases of synchronous GISTs and colorectal cancer (CRC) with immunohistochemistry analysis of c-Kit expression. This paper is also a review of the existing literature on the association of GISTs and CRC and the role of c-Kit in CRC.
Results
In the last 2 years, we observed two patients with synchronous CRCs and GISTs of the small bowel. The GISTs were incidentally discovered during the work-up for CRCs and excised at the time of the colon resection. Immunohistochemistry study did not reveal an expression of c-Kit in CRCs. Clinical implications of the association between these two neoplasms are described in this paper.
Conclusions
Synchronous CRC and GIST has been more frequently reported. Because of the limited number of cases, we cannot exclude an incidental relationship. The genetic pathways of tumorigenesis appear different for the two neoplasms. Further studies are needed to clarify a possible role of c-Kit in the development of colonic adenocarcinomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1983, Mazur and Clark coined the term gastrointestinal stromal tumor (GIST) to indicate a distinctive subgroup of gastrointestinal sarcomas [1]. Subsequently, Kindblom et al. identified the interstitial cell of Cajal, an intestinal pacemaker cell, as the origin of these tumors, further characterizing them as separate from intestinal sarcomas [2].
GISTs are now considered the most common mesenchymal tumors in the gastrointestinal tract. Over the last two decades, several changes have occurred in the diagnostics, treatment, and understanding of their pathogenesis. Although currently commonly diagnosed, little is known about their association with other gastrointestinal tumors.
The synchronous occurrence of primary colorectal adenocarcinomas (CRC) and GISTs has raised questions about a potential common origin and carcinogenetic mechanisms, and the possibility of similar treatment modalities. Furthermore, the association of specific tumors often leads to the discovery of novel genetic pathways to carcinogenesis that could be important for the development of oncologic treatments.
We present two cases of invasive CRCs and synchronous small-bowel GISTs. To investigate the possibility of a common genetic pathway between the synchronous CRCs and GISTs in these patients, immunohistochemical stains were performed for c-Kit.
C-Kit is a transmembrane protein encoded by the kit proto-oncogene, located on chromosome 4q11–q12; it acts as a receptor for the stem cell factor (SCF) growth factor and is found in virtually all GISTs. It is believed that its gain-of-function mutations may play a key role in the oncogenesis of these tumors [3, 4]. As c-Kit is recognized by the antibody CD117, its expression can be proven by immunohistochemistry methods.
Case reports
Case 1
An 86-year-old African American woman, in otherwise excellent general health, presented with right lower quadrant abdominal pain and weight loss. She had past histories of thyroidectomy and hysterectomy. A physical exam was unremarkable. Colonoscopy revealed yellow exudates at the appendiceal orifice but no mass. A computed tomography (CT) scan demonstrated an inflammatory mass in the right lower abdomen, with thickening of the cecum. Because of the persistence of abdominal symptoms, and the CT findings, an elective exploratory laparoscopy was performed. A loop of jejunum had a 4-cm pedunculated tumor that was adherent to the cecal mass. A laparoscopic assisted right hemicolectomy and a segmental small-bowel resection were performed.
Pathologic examination identified a moderately differentiated adenocarcinoma of the appendix that invaded through the muscularis propria and into the periappendiceal soft tissue, without lymph nodes being involved by the metastatic tumor (Stage IIA) [5]. In addition, a well-circumscribed polypoid mass arising in the muscularis propria of the jejunum was identified. The mass was a GIST with areas of necrosis and a mitotic count of 1/50 high power fields (hpf). Immunohistochemical studies showed the tumor cells stained strongly for CD117 and smooth muscle actin but showed no specific staining for desmin, CD21, CD34, and S-100. A satellite 0.8×0.5×0.5-cm lesion consistent with a GIST was also identified in the mesentery.
After an uneventful postoperative recovery, the patient remains cancer-free 6 months after her operation.
Case 2
A 67-year-old Chinese woman presented with new-onset bright red blood from the rectum. She was otherwise asymptomatic, and had a past medical history of hypertension and hypercholesterolemia.
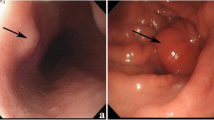
No masses were palpable on physical exam. Colonoscopy demonstrated a single, half-circumferential, ulcerated mass in the ascending colon. Biopsies of the mass were consistent with invasive adenocarcinoma. A staging CT scan of the abdomen and pelvis revealed a 5.4×3.6-cm soft tissue mass, which was presumed to be a metastatic lesion, in the left lower quadrant interlaced between small bowel loops (Fig. 1). The visualized loops of bowel were otherwise unremarkable without evidence of wall thickening or obstruction. There was no apparent liver disease.
Laparotomy confirmed the presence of a lesion in the ascending colon; a right hemicolectomy with a primary side-to-side, functional end-to-end stapled anastomosis was performed. An 8-cm pedunculated friable and erythematous lesion was also noted in the mid-jejunum (Fig. 2). There was no evidence of metastatic disease. A frozen section of the small bowel mass was consistent with a GIST. A small bowel resection with a primary end-to-end hand-sewn anastomosis was performed to remove the mass. Exploration of the abdomen revealed no additional lesions.
Final pathology identified the ascending colon mass as a moderately differentiated adenocarcinoma invading into the pericolonic adipose tissue without lymph node involvement (Stage IIA) [5]. The jejunal tumor was a GIST with three mitotic figures/50 hpf. Immunohistochemical analysis revealed strong diffuse staining of the tumor cells with CD117 c-Kit, while no specific tumor-cell staining was seen with S-100, desmin, smooth muscle actin, or muscle specific actin.
After an uneventful postoperative course, the patient is alive and free of recurrence 20 months after her operation.
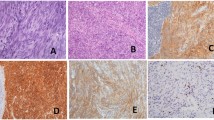
We performed an immunohistochemistry analysis to study the expression of c-Kit in the GIST and adenocarcinoma specimens from both patients described in our study. In both cases, strong c-Kit staining of GIST tumor cells, but no staining of CRC, was noted (Fig. 3).
Discussion
We have presented two cases of synchronous invasive CRC and small bowel GISTs that came to our attention over a period of less than 2 years. Despite the relative common occurrence of GISTs, reports of synchronous CRCs and GISTs are quite rare. In the experience of Kover et al., 7 of 43 patients with histologically proven GISTs were found with secondary neoplasm; three of these GISTs were colorectal adenocarcinomas [6]. Au et al. described 74 cases of GISTs diagnosed during a 5-year period; 23 of those were diagnosed incidentally, 19 of which were diagnosed during exploratory laparotomies for other tumors. One patient had an intestinal adenocarcinoma. Among the remaining 51 patients with symptomatic GISTs, 12 patients were also diagnosed with second tumors, including a single patient with a rectal adenocarcinoma. In the study by Au et al., up to 41% of the GISTs were associated with second malignancies, and 38% of these secondary malignancies were intestinal tumors [7].
The incidence of GIST in the United States is estimated to be 500 to 1,000 cases per year, excluding tumors less then 2 cm in size that are found incidentally at endoscopy or abdominal exploration [8]. CRC, on the other hand, is one of the most common cancers in the US, with approximately 150,000 new cases diagnosed every year, and with an individual's probability of developing a CRC in his/her lifetime at about 5.5% for women and 6% for men [9]. The possibility of an accidental association cannot be excluded, based on the available evidence.
Both colon cancer and GIST have a clear familial predisposition, excluding the well-known hereditary forms. Neither of the presented patients had a family history of gastrointestinal or other malignancies.
The genetic makeover of these two cancers has been thoroughly investigated. During progression from normal colonic epithelium to adenoma and carcinoma, several major genetic alterations occur [10]. Two major pathways have been identified in sporadic colorectal cancer: chromosomal instability, in up to 85% of the total cases, and microsatellite instability, in the remaining 15%. Interestingly, none of the most commonly involved genes in colorectal carcinogenesis (APC, DCC, p53, K-ras, DNA mismatch repair genes) have been identified to be associated in the development of GISTs. Rather, the vast majority of GISTs are associated with mutations of the proto-oncogene c-Kit, a tyrosine kinase receptor essential during embryonic development and postnatal life. Activation of c-Kit by its natural ligand, SCF, plays an important role in cellular transformation and differentiation, including cell proliferation and survival, adhesion, and chemotaxis [11].
Several studies have identified the presence of a c-Kit mutation in a wide variety of human malignancies besides chronic myeloid leukemia and GIST, including germ cell tumors, small cell lung carcinoma, neuroblastoma, melanoma, ovarian carcinoma, and breast carcinoma [12]. However, in most of these cancers the role of mutated c-Kit is not completely understood. It is reported that c-Kit kinase activity may induce cell proliferation in small cell lung cancer and in neuroblastomas; in tumors of breast, thyroid, and ovarian cancer, the malignant transformation and progression seem to associate with a loss of c-Kit protein expression [12].
Specifically in CRC, deregulated expression of c-Kit has been described. Bellone et al. reported overexpression of c-Kit and SCF in human CRC, and showed that c-Kit activation favors the growth, survival, migration, and invasive potential of DLD-1 colon carcinoma cells [13]. Attoub et al. identified the c-Kit receptor in human colon cancer cell lines HT29, HCT8/S11, and HCT116, and demonstrated inhibition of cellular proliferation and induction of apoptosis by STI571, a c-Kit tyrosine kinase inhibitor [14]. Toyota et al. demonstrated the expression of both c-Kit and SCF mRNA and c-Kit protein in colon carcinoma cell lines [15]. Sammarco et al. revealed c-Kit expression in up to 30% of CRC patients [12]. Other groups have reported that positive results of immunohistochemistry tests for c-Kit are quite rare in human CRC: Singer et al. showed expression of c-Kit in only 4 of 72 (6%) biopsies of CRC [16]. Reed et al. observed high cytoplasmic c-Kit staining in only 1.6% of the CRC patients evaluated in their study [17]. The cases in our study had c-Kit-positive GISTs, but, for the synchronous CRC, tests for c-Kit were negative with absent staining (Fig. 3).
Surgery remains the mainstay of therapy for both nonmetastatic GISTs and CRC, although the operative strategies and extents of resection are fairly different [18]. If an incidental GIST is found at laparotomy, the lesion should be treated as malignant. Because skip metastases have not been reported in GIST, a wide resection is not indicated. Furthermore, microscopic involvement of resection margins does not appear to affect survival [18, 19]. Given the rarity of lymph-node involvement, routine lymphoadenectomy is not currently recommended [20]. In contrast, curative resection for colorectal cancer requires the inclusion of lymphatic station with the specimen, as well adequate microscopic radial and distal margins. GIST and CRC are further differentiated by their response to adjuvant treatments and their susceptibility to single or multidrug regimens.
Conventional chemotherapy and radiation appear to have no impact on the natural history of GISTs [20–23]. Rather, c-Kit constitutes an excellent molecular target for therapeutic interventions; imatinib mesylate (STI571), a selective tyrosine kinase competitive inhibitor, appears to be an effective drug. A US–Finland study and a trial by the European Organization for Research and Treatment of Cancer reported similar partial response rates (53.7% and 52.7%, respectively) in treatment of metastatic or locally advanced disease [24, 25]. Adjuvant conventional systemic chemotherapy outside controlled trials is currently not recommended due to the very limited evidence of any efficacy of this approach.
With the notable exception of GISTs, chronic myeloid leukemia, and glioblastomas, clinical trials involving the use of tyrosine kinase inhibitors against other tumors have demonstrated that the targeting of a single tyrosine kinase is not sufficient to produce a clinically meaningful response [16]. To our knowledge, imatinib mesylate has not been tested for the treatment of CRC; the lack of evidence of a key role of c-Kit in the development of CRC and the rarity of its mutation in those tumors do not support its routine use for the treatment of CRC.
The natural history of GIST is characterized by a high rate of recurrence (up to 40% within 2 years) [18]. Much like CRC, GISTs preferentially metastasize to the liver, as well as spread locally to peritoneal surfaces, and half of the cases of locoregional relapse are accompanied by liver metastasis [26]. In contrast, extra-abdominal metastases are uncommon [19, 26, 27]. While the resectability of primary disease is, on average, 70%, fewer then one third of recurrent cases are amenable to macroscopically complete surgical extirpation [18, 19].
Overall survival after complete resection of GIST ranges between 48% and 65% at 5 years, and tends to be longer in patients with low-grade tumors (100% at 10 years for tumors with 0–1 mitosis/30 hpf); high-grade lesions (>10 mitosis/10 hpf) have the worst outcome (0% survival at 10 years); nonetheless, the absence of a high mitotic index does not guarantee a benign outcome [28]. Overall, a 5-year survival for CRC is more reliably predictable, correlates mainly with the preoperative staging, and ranges from 4–7% for stage IV to 90% for stage I [29].
Well-defined follow-up protocols for patients with GISTs are not available; however, several reports have recently underlined the usefulness of CT scans in the evaluation of the tumor response to imatinib mesylate. Specifically, “cystic-like” transformation of hepatic metastases may indicate good partial response, while a solid nodule inside a cystic mass seems a reliable sign of regrowth [30]. Other studies have suggested that a positron emission tomography scan that shows a decrease in 18F-fluorodeoxyglucose uptake after the start of treatment with imatinib mesylate may indicate a positive treatment result and a prolonged progression-free survival [31–33]. The exact role of CT scans and their frequency during follow-up after surgery for CRC are undefined.
Conclusion
The association of CRC and GIST is an uncommon occurrence. Given the high incidence of CRC and the relative frequency of GIST, we cannot exclude an incidental relationship based on the available data.
The known genetic pathways of tumorigenesis are different for the two neoplasms; c-Kit appears to be occasionally overexpressed in CRC, and it is not clear if the protein is indeed a key player in the carcinogenetic process, as it is in GISTs. Several papers have shown that STI571 may inhibit the in vitro growth of CRC cell lines. These encouraging results warrant further preclinical investigations of c-Kit expression in colon cancers to assess if and when the use of newer tyrosine kinase inhibitors in a multimodality regimen could be considered in CRC.
References
Mazur MT, Clark HB (1983) Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 7:507–519
Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM (1998) Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152:1259–1269
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580
Rossi CR, Mocellin S, Mencarelli R, Foletto M, Pilati P, Nitti D, Lise M (2003) Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer 107:171–176
Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M (2002) AJCC cancer staging manual, 6th edn. Springer, Berlin Heidelberg New York, pp 113–123
Kover E, Faluhelyi Z, Bogner B, Kalmar K, Horvath G, Tornoczky T (2004) Dual tumours in the GI tract: synchronous and metachronous stromal (GIST) and epithelial/neuroendocrine neoplasms. Magy Onkol 48:315–321
Au WY, Ho KM, ShekTW (2004) Papillary renal cell carcinoma and gastrointestinal stromal tumor: a unique association. Ann Oncol 15:843–844
DeMatteo RP, Brennan MF (2004) Gastrointestinal stromal tumors. In: Cameron JL (ed) Current surgical therapy, 8th edn. Elsevier Mosby, Philadelphia, pp 100–103
Welton ML, Varma MG, Amerhouser A (2001). Colon, rectum, and anus. In: Norton JA, Bollinger RR, Chang AE, Lowry SF, Mulvihill SJ, Pass HJ, Thompson RW (eds) Surgery: basic science and clinical evidence. Springer, Berlin Heidelberg New York, pp 667–762
Hahn M, Koufaki ON, Schackert HK (1998) Molecular biology of colorectal cancer and clinical consequences for colorectal cancer syndromes. Langenbecks Arch Surg 383:389–396
Linnekin D (1999) Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol 31:1053–1074
Sammarco I, Capurso G, Coppola L, Bonifazi AP, Cassetta S, Delle Fave G, Carrara A, Grassi GB, Rossi P, Sette C, Geremia R (2004) Expression of the proto-oncogene c-KIT in normal and tumor tissues from colorectal carcinoma patients. Int J Colorectal Dis 19:545–553
Bellone G, Carbone A, Sibona N, Bosco O, Tibaudi D, Smirne C, Martone T, Gramigni C, Camandona M, Emanuelli G, Rodeck U (2001) Aberrant activation of c-kit protects colon carcinoma cells against apoptosis and enhances their invasive potential. Cancer Res 61:2200–2206
Attoub S, Rivat C, Rodrigues S, Van Bocxlaer S, Bedin M, Bruyneel E, Louvet C, Kornprobst M, Andre T, Mareel M, Mester J, Gespach C (2002) The c-Kit tyrosine kinase inhibitor STI571 for colorectal cancer therapy. Cancer Res 62:4879–4883
Toyota M, Hinoda Y, Takaoka A, Makiguchi Y, Takahashi T, Itoh F, Imai K, Yachi A (1993) Expression of c-kit and kit ligand in human colon carcinoma cells. Tumour Biol 14:295–302
Singer CF, Hudelist G, Lamm W, Mueller R, Czerwenka K, Kubista E (2004) Expression of tyrosine kinases in human malignancies as potential targets for kinase-specific inhibitors. Endocr Relat Cancer 11:861–869
Reed J, Ouban A, Schickor FK, Muraca P, Yeatman T, Coppola D (2002) Immunohistochemical staining for c-kit (CD117) is a rare event in human colorectal carcinoma. Clin Colorectal Cancer 2:119–122
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Pierie J, Choudry U, Muzikansky A, Yeap B, Souba W, Ott M (2001) The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 136:383–389
Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF (2000) Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 7:705–712
McGrath PC, Neifeld JP, Lawrence W, Kay S, Horsley JS 3rd, Parker GA (1987) Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 206:706–710
Dougherty MJ, Compton C, Talbert M, Wood WC (1991) Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic group by mitotic count. Ann Surg 214:569–574
Conlon KC, Casper ES, Brennan MF (1995) Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Onc 2:26–31
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480
Van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, Silberman S, Nielsen OS (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358:1421–1423
Mudan SS, Conlon KC, Woodruff JM, Lewis JJ, Brennan MF (2000) Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer 88:66–74
Eilber FC, Rosen G, Forscher C, Nelson SD, Dorey F, Eilber FR (2000) Recurrent gastrointestinal stromal sarcomas. Surg Oncol 9:71–75
Nowain A, Bhakta H, Pais S, Kanel G, Verma S (2005) Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol 20:818–824
Efron J, Wexner SD (2001). Rectal cancer. In: Cameron JL (ed) Current surgical therapy, 7th edn. Elsevier Mosby, Philadelphia, pp 235–245
Vanel D, Albiter M, Shapeero L, Le Cesne A, Bonvalot S, Le Pechoux C, Terrier P, Petrow P, Caillet H, Dromain C (2005) Role of computed tomography in the follow-up of heptic and peritoneal metastases of GIST under imatinib mesylate treatment: a prospective study of 54 patients. Eur J Radiol 54:118–123
Van den Abbeele AD, Badawi RD (2002) Use of positron emission tomography in oncology and its potential role to assess response to imatinib mesylate therapy in gastrointestinal stromal tumors (GISTs). Eur J Cancer 38:S60–S65 (Suppl 5)
Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R, Dumez H, Silberman S, Mortelmans L, van Oosterom A (2003) 18FDG-positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer 39:2012–2020
Goerres GW, Stupp R, Barghouth G, Hany TF, Pestolazzi B, Dizendorf E, Schnyder P, Luthi F, von Schulthess GK, Leyvraz S (2005) The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumors: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging 32:153–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melis, M., Choi, E.A., Anders, R. et al. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor (GIST). Int J Colorectal Dis 22, 109–114 (2007). https://doi.org/10.1007/s00384-006-0089-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-006-0089-6