Abstract
Objectives
The aim of this study was to assess the results of laparoscopic surgery for rectal carcinoma (LSRC) during the learning curve throughout the introduction of this technique at our medical center.
Materials and methods
From January 2003 to April 2004, 40 patients undergoing surgery were assigned to laparoscopic surgery group (LSG) (n=20) or conventional surgery group (CSG) (n=20). Data were prospectively collected to statistically analyze clinical, anatomopathological, and economic variables.
Results
Groups were comparable in age, sex, body mass index, American Society of Anesthesiologists score, surgical technique performed, tumor size and distance, Dukes’ stage, and proportion of patients with previous abdominal surgery and radiotherapy. There was no difference in operative time. LSG blood loss was lower (p<.0001). LSG peristalsis and oral intake began earlier (p<.0001). LSG hospital stay was shorter (p<.0001). Intraoperative complications (10% LSG vs 15% CSG) and overall morbidity (35% LSG vs 45% CSG) were no different. LSG did not record any anastomotic leakages. Two patients (10%) were converted to open surgery. Regarding oncologic adequacy of resection, specimen length and number of nodes harvested were no different. LSG distal and radial resection margins were greater (p<.0001; p=.03). LSG operative costs were greater (p<.0001). However, CSG hospitalization costs were higher (p<.001). There was no overall difference (p=0.1).
Conclusions
LSRC has been a reliable and efficient technique during the learning curve at our hospital.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preoperative radiotherapy and meticulous surgical techniques have been proved to be key tools in reducing local recurrence of rectal cancer [1, 2].
After some years of controversy, experimental research on one hand and survival and long-term recurrence rates at health centers of excellence on the other have validated laparoscopy in the treatment of colorectal cancer [3–5].
Surgical technique for rectal cancer must include the following principles, independent of the chosen approach: en bloc resection of tumor and lymphatic tissue, no-touch technique, and total mesorectal excision for middle and lower third lesions.
The recent introduction of endoscopic techniques for curative treatment of rectal carcinoma has not yet allowed results during the learning curve and in real working conditions to be validated.
To assess reliability and efficiency of laparoscopy in the curative treatment of rectal carcinoma during the learning stage, at our university hospital, we compared short-term results of laparoscopic surgery for rectal cancer (LSRC) with those obtained by conventional surgery.
Materials and methods
Between January 2003 and April 2004, 44 patients (24 women and 20 men) with rectal cancer were evaluated for study inclusion.
Patients were randomly assigned to the laparoscopic surgery group (LSG) or conventional surgery group (CSG) by the Outpatient Services Department at our medical center.
Laparoscopic surgery (LS) was performed by a team established in November 2002 to implement laparoscopic colorectal cancer surgery in our department. This team comprised three surgeons, two of whom were experienced in advanced laparoscopic surgery and open colorectal surgery, and the third was specifically trained at health centers of excellence for laparoscopic colorectal surgery (Hospital Clínico de Barcelona/Center of Laparoscopic Surgery of South Florida). All laparoscopic procedures were performed with two team members acting as primary surgeon and first assistant. A three-surgeon team, with more than 5-years experience in conventional rectal cancer treatment and who regularly perform this type of surgery, performed the operations on patients assigned to conventional surgery (CS).
Tumors with obstruction or perforation symptoms, preoperatively diagnosed T4 staging, tumors larger than 7 cm and local surgery candidates were excluded from the study.
Patient’s preoperative routine included physical examination during medical appointment, analyses including liver function tests, chest x-rays and electrocardiograms, carcinoembryonic antigen, abdominal computed tomography (CT) to assess liver involvement, and pelvic magnetic resonance imaging (MRI) and/or endorectal ultrasound to assess the degree of local infiltration.
Carcinomas were included when located less than 15 cm from the anal verge, measured on removal of a flexible endoscope.
Patients with preoperatively staged T3 or T4 middle and lower third tumors or mesorectal adenopathy without distant metastases received a regimen of preoperative radiotherapy (45 Gy in 4 weeks).
All patients were prepared with a lavage solution the day prior to surgery and received antibiotic prophylaxis during anesthetic induction.
Surgical technique
Patients were operated under general anesthesia and placed in the Lloyd Davis position, with urinary catheterization in all cases.
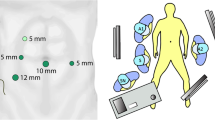
LSG patients had four trocars placed: two 5-mm trocars, one on either side of the umbilicus, at the right iliac fossa and left abdominal flank; a third 10-mm trocar for the camera at the umbilicus; and a fourth 12-mm trocar for linear stapler insertion close to the right anterosuperior iliac spine. In addition, a fifth subxiphoid trocar was placed when mobilizing the splenic flexure of the colon was required and a sixth suprapubic trocar for lower rectal dissection.
In all cases, colon and rectum dissection was performed in the medial–lateral direction, using an ultrasonic scalpel, opening a keyhole in the mesocolon to identify left ureter and gonadic vessels, taking special care to preserve dorsolumbar sympathetic nerve terminals, followed by ligation of the inferior mesenteric artery and vein at its origin 1 cm close to the aorta and splenic vein using a vascular linear stapler. The rectum and mesorectum were mobilized circumferentially from their avascular plane to between 1 and 2 cm below the levator muscle, sparing the pelvic fascia and autonomic nerve plexus. Total mesorectal excision (TME) was performed on all tumors located in the middle or lower thirds. Distal section of the rectum was performed with a 45-mm linear stapler, using two firings in most cases. A protected Pfannenstiel incision was performed for specimen extraction. Passage reconstruction was carried out intracorporeally with a transanal circular (end-to-end, EEA) stapler. Protective ileostomy was performed in low anterior resections with stapling problems and in those cases where the saline instillation test detected air leakage.
In abdominoperineal resections, a sigmoid colostomy was performed, and the specimen was delivered via the perineal route.
All patients had low pressure suction drainage at the pelvis.
In the CSG group, the technique performed followed procedures for treatment of rectal cancer as established by surgical societies. TME criteria were identical to those in the LSG.
Demographic, clinical, anatomopathological, and economic data variables for both groups were prospectively collected into a database. Variables regarding patient characteristics included age, sex, American Society of Anesthesiologists (ASA) score, body mass index (BMI), previous abdominal surgery, and radiotherapy records. Clinical variables comprised operative data, operative time, blood loss and conversion rates, as well as data regarding return of peristalsis, introduction of oral intake, average hospital stay and perioperative transfusion. Data regarding perioperative morbidity, as well as readmission and reoperation were also collected. Anatomopathological data such as specimen length, number of lymph nodes harvested, and radial and distal margins were analyzed. Procedure cost per patient (surgical plus hospitalization costs) was also studied.
Quantitative variable results are expressed in mean/standard deviation. Categorical variable results are expressed as percentages. Variables studied were age, sex, tumor size and location, BMI, ASA score, and previous abdominal surgery.
Group comparisons (LSG/CSG) for quantitative variables were carried out using Student’s t test. Group comparisons (LSG/CSG) for categorical variables were carried out with Chi-square or Fisher’s exact test.
Statistical analyses were carried out using the SPSS 11.0.1 statistical package.
Results
Four patients were ineligible for the study. Of these, two had preoperatively staged T4 tumors, one T1 candidate was assigned to local surgery, and one tumor was larger than 7 cm. Finally, 40 patients were included. Patient characteristics are summed up in Table 1. Both groups were comparable in age, sex, ASA score, surgical technique performed, tumor size and distance to anal verge, tumor–node–metastases (TNM) staging, and proportion of patients having previously undergone abdominal surgery and radiotherapy.
Operative times were no different. Blood loss was lower in the LSG. Return of intestinal activity and introduction of oral intake took place earlier in the LSG. LSG hospital stay was shorter. Despite a greater proportion of CSG patients requiring blood transfusions (35 vs 65%, p=.06), this difference was not statistically significant. Two LSG patients (10%) required conversion, in one case owing to difficulty exposing the surgical field due to obesity and in the other to tumor size (Table 2).
No perioperative deaths were recorded. Intraoperative complication rates were no different, neither for overall patient postoperative complication rate (35 vs 45%) nor in readmission or reoperation rates. However, the LSG registered higher urodynamic disorder rates: three cases of acute urine retention and one of incontinence, which required temporary urinary catheterization, against zero cases in the CSG (Table 3).
Various parameters were examined to check adequacy of oncological resection. There were no differences in the proportion of patients with curative surgery or in the percentage of patients with tumor-free margins. One case of tumor perforation was registered in the LSG, during perineal dissection. Regarding histological assessment of surgery, neither the length of surgical resection nor the number of lymph nodes harvested was different. The LSG had a higher number of tumors with radial margins greater than 2 mm, as well as greater distal and radial resection margins (Table 4).
As for economic variables studied, operative costs were greater in the laparoscopy group. However, hospitalization costs were greater in the open surgery group. Overall costs per procedure were no different (Table 5).
Discussion
Randomized prospective research has proved the safety of laparoscopy for treatment of colon cancer, in terms of long-term survival and recurrence [3–5]. Although there is not much scientific evidence regarding rectal resections for carcinoma, several prospective nonrandomized studies point in the same direction [6, 7]. Altogether, this has meant that endoscopic techniques in general surgery units are indeed a reality. The learning curve for LSRC, however, is yet to be defined, and its results, regarding the standards of excellence these procedures must attain, are yet to be validated [8].
LSRC does offer advantages over conventional surgery: pain reduction, earlier introduction of oral intake, and reduced hospital stay, at the expense of longer operative times [9]. However, we have not found differences at our institution in operative time between both techniques. The difference in hospital stay was due to later introduction of oral intake and a hospital stay of over 30 days in the two CSG patients with postoperative anastomotic leakages.
As a result of lower blood loss during laparoscopic surgery, the need for perioperative transfusions is greater in open surgery [10]. In our patients, this difference is close to statistical significance.
Postoperative morbidity rates reported in literature vary from 18 to 44%, most comparative studies not having encountered differences between both approaches [11]. The most common complications are surgical wound infections, at around 10–15% for anterior resections and above 25% for abdominoperineal resections [12]. Some authors found a higher number of short- and long-term wound complications in laparoscopic surgery [13]. In our study, the auxiliary incision was protected by means of a plastic bag from specimen extraction until closure of the intestinal lumen, gloves, and instruments being changed for wound closure and subcutaneous tissue washed out with 5‰ povidone–iodine solution. Perineal incisions received partial closure with a subcutaneous drain. Despite all measures taken, we did not find a significant difference between both groups.
Frequency of anastomotic leakage in open surgery is reported at around 10%, independent of the proportion of patients who received neoadjuvant treatment or who had an ostomy [14]. These rates are similar to those obtained with laparoscopic surgery [12]. Incidence of anastomotic leakage has been related to the distance at which anastomosis is performed, independent of the chosen approach [15, 16]. At our hospital, we systematically checked anastomotic integrity in all anterior resections by instillation of saline into the pelvis, with air insufflation through the rectum before and after performing anastomosis. There were no cases of suture dehiscence in the LSG. The CSG recorded two cases of postoperative anastomotic leakage in low anterior resections. One of them required Hartmann’s procedure after presenting with apparent sepsis and peritonitis. The second case, who presented with fever and pelvic collection, had an ileostomy and did not require reoperation.
Urodynamic disorders during the immediate postoperative period were higher in the LSG (Table 3). These complications, although not always commented in literature, have often been related to rectal cancer, related factors being abdominoperineal resection, patient age over 60 years, and a background of prostate disorders, with rates spanning from 10 to 60%, depending on references [17]. In our experience, laparoscopy allows excellent pelvic and autonomic nerve vision, although lateral and anterior dissection in the correct plane is complicated. Learning of the technique and early removal of urinary catheterization in laparoscopic surgery might account for the higher incidence of urodynamic disorders in this group.
When surgery is performed correctly, there is no difference in number of resected lymph nodes [18]. The subject of safety margins is more controversial and has been studied by various authors. Tate et al. [19] obtained a lower distal margin in 11 laparoscopically operated patients in comparison with an open surgery group of 14 patients, despite greater anal verge distance in the laparoscopy group. However, Lord et al. [20] found differences in favor of laparoscopic surgery, 4.9 vs 2.5 cm, although average tumor distance in each group was not specified. In more recent research, distal resection margins obtained are in the region of 3–4 cm [6, 21, 22], no different to those obtained by open surgery. In addition to problems with distal margins, Darzi et al. [23] noted a reduction of approximately 50% in the tumor-free lateral margin in a group of 12, compared with 16 patients who underwent abdominoperineal resection by laparotomy. Differences have not been observed in radial margins either, which fluctuate between 0.6 and 0.8 cm, although there has been less research in this area.
Recently, MRC-CLASICC trial has addressed this issue, with similar rates of positive resection margin between treatment groups [24].
Although oncological result evaluation is carried out using local recurrence and mortality rates due to cancer, some of our research results in respect of oncologic adequacy of resection are relevant. In LSG, surgery was curative in 17 cases, 2 patients revealed synchronous liver metastases and a third rectal tumor perforation, encountered at the time of perineal dissection. In the CSG, 14 patients underwent curative surgery, 4 had liver metastases and, in another, a radial margin of more than 2 mm was not achieved. The proportion of patients with a radial margin more than 2 mm was larger in LSG (100 vs 80%, p=.04), as were the radial (0.7 vs 1.2 cm, p=.03) and distal (3.7 vs 1.7 cm, p=.0001) margins of resection. In relation to these differences, we believe that the impossibility of palpating the tumor in LS leads to performing upper rectal tumor dissection to an area accessible for rectal palpation, which could account for greater distal margins in the LSG. Further, in our opinion, the higher proportion of patients with D stage tumors in the CSG explains the radial margin results referred. Other important and controversial issue is the number of low number of lymph nodes harvested in our patients (mean 6.5). According to the TNM guidelines, ideally 12 lymph nodes should be routinely examined. However, the number of 12 nodes is not a requirement and usually hard to reach. An important TME trial found that in 82% of the cases, less than 12 lymph nodes were examined (mean 7.5) [25].
One of the inconveniences ascribable to LSRC is the increase in costs owing to surgical intervention, mainly owing to disposable materials. Some recent research demonstrates that this expense is offset by the reduction of indirect costs incurred by hospital stay, pharmaceuticals, laboratory, and nursing [15, 16]. In our research, there was no difference in overall procedure costs.
The main problem for implementing LSRC is the more or less prolonged learning curve required, which does not guarantee accomplishment of the standards of quality established for this type of surgery. Following the results obtained in our experience, we believe that it is crucial that the team performing this type of surgery during the learning stage has a combination of thorough experience in open colorectal surgery and advanced laparoscopy techniques, together with specific LSRC training obtained at referral hospitals. In these conditions, our results suggest that laparoscopic surgery can be performed safely during the learning curve, reaping its clinical benefits, without compromising the oncologic principles of resection or increasing procedure costs.
References
Dahlberg M, Glimelius B, Pahlman L (1999) Improved survival and reduction in local failure rates after preoperative radiotherapy: evidence for the generalizability of the results of Swedish rectal cancer trial. Ann Surg 229:493–497
Heald RJ, Husband EM, Ryall RDH (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Lacy AM, García Valdecasas JC, Delgado S, Castells A, Taura P, Piqué JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 29:2224–2229
Clinical Outcomes of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 20:2050–2059
Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, Morino M, Lacy AM, Colon Cancer Laparoscopic or Open Resection Study Group (COLOR) (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Morino M, Parini U, Giraudo G, Salval M, Contul RB, Garrone C (2003) Laparoscopic total mesorectal excision. A consecutive series of 100 patients. Ann Surg 237:335–342
Tsang W, Cheng C, Li M (2003) Prospective evaluation of laparoscopic total mesorectal excision with colonic J-pouch reconstruction for mid and low rectal cancer. Br J Surg 90:867–871
Ortiz H (2003) Estándares de calidad e instrumentación necesaria en la cirugía del cáncer de recto bajo. Cir Esp 74:321–324
Chen HH, Wexner SD, Weiss EG, Nogueras JJ, Alabaz O, Iroatulam AJ, Nessim A, Joo JS (1998) Laparoscopic colectomy for benign colorectal disease is associated with a significant reduction in disability as compared with laparotomy. Surg Endosc 12:1397–1400
Kiran R, Delaney C, Senagore A, Millward B, Fazio V (2004) Operative blood loss and use of blood products after laparoscopic and conventional open colorectal operations. Arch Surg 139:39–42
Pitarsky A, Rosenthal R, Weiss E, Wexner S (2002) Laparoscopic total mesorectal excision. Surg Endosc 16:558–562
Yong L, Deane M, Monson J, Darzi A (2001) Systematic review of laparoscopic surgery for colorectal malignancy. Surg Endosc 15:1431–1439
Winslow E, Fleshmen J, Birnbaum E, Brunt L (2002) Wound complications of laparoscopic vs open colectomy. Surg Endosc 16:1420–1425
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer Group (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR (1997) Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg 185:105–113
Scheidbach H, Schneider C, Konradt J, Barlehner E, Kohler L, Wittekind Ch, Kockerling F (2002) Laparoscopic abdominoperineal resection and anterior with the curative intent for carcinoma of the rectum. Surg Endosc 16:7–13
Quah HM, Jayne DG, Eu KW, Seow-Choen F (2002) Bladder and sexual dysfunction following laparoscopically assisted and conventional open mesorectal resection for cancer. Br J Surg 89:1551–1556
Zmora O, Wexner S, Saber A (2001) Laparoscopy surgery for colon and rectal cancer. Curr Probl Cancer 25:283–309
Tate J, Kwok S, Dawson J, Lau W, Li A (1993) Prospective comparison of laparoscopic and conventional anterior resection. Br J Surg 80:1396–1398
Lord S, Larach S, Ferrara A, Williamson P, Lago C, Lube M (2000) Laparoscopic resections for colorectal carcinoma: a three-year experience. Hepatogastroenterology 47:683–691
Wen-Xi W, Yao-Min S, Yi-Bin H, Li-Zong S (2004) Laparoscopic versus conventional open resection of rectal carcinoma: a clinical comparative study. World J Gastroenterol 10:1167–1170
Feliciotti F, Guerrieri M, Paganini A, De Sanctis A, Campagnacci R, Perretta S, D’Ambrosio G, Lezoche G, Lezoche E (2003) Long-term results of laparoscopic vs open resections for rectal cancer for 124 unselected patients. Surg Endosc 17:1530–1535
Darzi A, Lewis C, Menzies-Gow N, Guillou P, Monson J (1995) Laparoscopic abdominoperineal resection. Surg Endosc 9:414–417
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC Trial Group (2005). Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW, van Krieken JH, Pathology Review Committee and the Cooperative Clinical Investigators (2001) No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 19:1976–1984
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arteaga González, I., Díaz Luis, H., Martín Malagón, A. et al. A comparative clinical study of short-term results of laparoscopic surgery for rectal cancer during the learning curve. Int J Colorectal Dis 21, 590–595 (2006). https://doi.org/10.1007/s00384-005-0057-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-005-0057-6