Abstract
Background
Stenoses and fistulas are frequent complications in patients with Crohn’s disease (CD). They represent a major diagnostic and therapeutic challenge and surgical intervention is often required. The availability of novel, anti-TNF strategies for therapy has raised the question as to what extent these new treatment options have impact on the clinical decision-making process regarding the necessity for surgery.
Discussion
A short overview of the current pathophysiological understanding of CD, focusing on the immunology of the intestinal mucosa, is given. Then the problems of proper clinical management of stenoses and fistulas are addressed. With regard to symptomatic stenoses, attention will be given to novel diagnostic tools for the distinction between inflammatory and fibrotic stenoses, and our clinical experience with the treatment of symptomatic inflammatory stenoses with infliximab will be discussed. With regard to fistulizing CD, the data that are currently available for medical therapy are summarized with special reference to the studies on the efficacy of anti-TNF treatment.
Conclusion
With regard to moderately and severe inflammatory stenoses, medical treatment with infliximab may be an option after careful assessment of the inflammatory nature of the stenosis and exclusion of a septic focus. With regard to fistulas, anti-TNF treatment is a valuable option that is likely to improve the clinical outcome. Based on the available data, however, anti-TNF treatment cannot yet replace surgical intervention when necessary. Prospective trials of medical therapy and a combination of medical and surgical therapy for complex fistulas and internal fistulas are needed to define the potential and the limitations of these novel therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) of unknown origin characterized by a chronic, granulomatous, transmural inflammation that can affect the entire gastrointestinal tract with a discontinuous pattern. The prevalence of CD has increased in Western countries over the past decades and mainly young patients are affected, with a peak incidence between the ages of 15 and 35 years [1]. Chronic complications such as fissurae, fistulas with abscesses, stenoses, and strictures are frequent and affect approximately 40% of all patients with CD [2, 3].
The etiology of CD is still unclear and should be considered as multi-factorial according to recent studies [4]. Among genetic factors, mutations in the recently identified NOD2 gene could account for the development of CD in a subgroup of patients [5, 6]. NOD2 seems to play a role in modulating the innate immune response following stimulation by bacterial antigens. Environmental and infectious factors play a role as well. Considerable progress in our pathophysiologic understanding of CD, however, has been achieved by the investigation of regulatory mechanisms of the intestinal immune system in CD.
Mucosal surfaces such as the intestinal mucosa are special interfaces for the interaction between the organism and its environment. They possess a specialized mucosal immune system (e.g., the gut associated lymphoid tissue, GALT). Due to its large surface the gut can be regarded as the biggest immune organ of the human body. In the gut, the organism is physiologically exposed to a large amount of antigens from natural flora and food. Whereas a systemic immune response to these foreign antigens should be prevented in order not to damage the organism, potentially pathogenic antigens have to be recognized and eliminated at a local level. The intestinal epithelium with the mucus offers some protection, but in addition, it is the accomplishment of the GALT to keep this fragile immunological balance between hyporesponsiveness and efficient immune defense [7].
In this review, we will summarize recent evidence of the role of anti-TNF strategies in CD. Special attention will be given to the administration of anti-TNF antibodies in patients with stenosing or fistulizing CD.
Clinical therapy of Crohn’s disease
Important immunological factors have been identified for the pathogenesis of CD, including cellular components such as monocytes/macrophages and CD4+ T-lymphocytes, as well as humoral components such as TNF-α, IL-12, IL-18 and others. One of the best characterized cytokines is TNF-α [8]. There is a large amount of clinical and experimental data that shows a pivotal role of TNF-α in the pathogenesis of CD. The reproducibility of the pathogenetic role of TNF-α in various animal models of chronic colitis, together with the availability of modern biomedical techniques, made it possible to develop and test novel anti-TNF strategies such as the chimeric anti-TNF-antibody infliximab in CD patients. Infliximab is a genetically engineered IgG1 murine-human monoclonal antibody with a constant region of human IgG1k-immunoglobulin and a variable region of a monoclonal mouse anti-human antibody [9, 10]. Infliximab binds both soluble and membrane-bound TNF-α and most likely blocks the interaction of TNF-α with the TNF-receptors in this way [11]. Ex vivo studies showed that TNF-blockade by infliximab may lead to an elimination of blood monocytes by induction of programmed cell death (apoptosis) [12].
Conventional medical treatment options for CD have included corticosteroids and 5-aminosalicylic acid, which can help to achieve clinical remission. Unfortunately, however, relapses may occur after a few weeks. The utilization of immunosuppressive drugs, especially of the Rac1 inhibitor azathioprine, has increased long-term remission rates from 33 to 54% with a concomitant steroid sparing effect [13–18]. However, these outcome data refer to treatment of patients with active CD in general, irrespective of the particular type of disease, and do not apply to patients with fistulizing disease. To date, only a few controlled trials on medical therapy have addressed the responsiveness of fistula healing specifically (see below).
Crohn’s disease represents a major challenge for surgery. With increasing knowledge and experience in the treatment of CD, there has been a change in the philosophy regarding the surgical treatment of CD [19, 20]. Emergency surgical intervention is mandatory in cases of fulminant colitis with sepsis, toxic megacolon, severe bleeding, abscesses or perforation. However, long-term follow-up studies have shown that relapses of CD, even after extensive surgery, are relatively frequent. The probability of achieving definite healing of CD by surgery alone is small and repeated surgical interventions may lead to short bowel syndrome. Therefore, the optimal treatment of patients with CD requires an interdisciplinary approach, although the decision for a necessary or even emergency surgery must not be delayed. However, the primary goal of a close collaboration between gastroenterologists and surgeons should be to anticipate potential problems in order to minimize emergency interventions.
Stenoses in Crohn’s disease and anti-TNF strategies
General principles exist regarding the medical management of stenoses and strictures. Due to their fibrotic nature, strictures are unlikely to respond to anti-inflammatory medical treatment. In contrast, acute inflammatory stenoses during flares should be treated medically, unless there is evidence of abscesses. However, even inflammatory stenoses bare the risk of ileus, perforation or peritonitis when severe complaints suggest a subtotal or even total obstruction of the lumen and, therefore, may require surgical treatment as well. A new therapeutic option in stenosing CD represents the recombinant anti-TNF-antibody infliximab that has been shown to induce rapid anti-inflammatory effects with symptomatic improvement [21, 22]. Infliximab might thus meet the need for medical treatment with rapid effects in symptomatic inflammatory stenoses. However, there are few data from prospective studies for this particular indication. In addition, the proper distinction between inflammatory stenosis and fibrotic stricture prior to treatment represents a major diagnostic challenge. Due to the involvement of all layers of the bowel wall in CD, an inflammatory process of the outer layers may be missed endoscopically. Thus, additional techniques are required to unequivocally reveal the inflammatory nature of a stenosis.
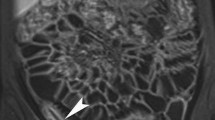
Hydro-magnetic resonance imaging (hydro-MRI), using 2.5% mannitol solution for oral bowel opacification and gadolinium-DTPA as intravenous contrast medium, represents a valuable diagnostic tool to determine the inflammatory or fibrotic nature of a stenosis with high specificity and sensitivity (Figs. 1, 2) [23, 24]. However, hydro-MRI is fairly cost intensive and local expertise for proper assessment of hydro-MRI pictures is a prerequisite. Another potentially interesting tool in this context is the 18-F-fluoro-deoxy-glucose-positron-emission-tomography (PET) [25, 26]. This method is based on the observation that inflamed tissue has an increased metabolism with an increased glucose turnover. After intravenous application of fluorine-18-marked fluoro-2-deoxy-d-glucose (FDG), FDG accumulates in the inflamed area of the bowel (Fig. 3). In a pilot study, this method displayed good sensitivity and specificity to reveal inflammatory activity in stenotic bowel segments [27]. However, further prospective studies are required to prove the validity of this approach.
Hydro-MRI of a Crohn’s patient with inflammatory stenosis of the left transverse colon prior to anti-TNF treatment (courtesy of [28]). a Lack of intraluminal contrast after oral bowel opacification with 2.5% mannitol as a result of massive bowel wall thickening with prestenotic dilatation (T2-weighted). b Massive enrichment of gadolinium-DTPA as intravenous contrast medium in the wall of the left transverse colon indicating inflammation (T1-weighted)
Hydro-MRI of the same Crohn’s patient as in Fig. 1 following anti-TNF treatment (courtesy of [28]). a Normal intraluminal filling with 2.5% mannitol in the transverse colon indicating complete regression of the stenosis, and no prestenotic dilatation (T2-weighted). b Minimal enrichment of gadolinium-DTPA in the wall of the left transverse colon indicating healing of the transmural inflammation (T1-weighted)
An fluorine-18-marked fluoro-2-deoxy-d-glucose-positron emission tomography (FDG-PET) of a Crohn’s patient with inflammatory stenosis of the neoterminal ileum following ileoascendostomy. Increase and decrease in FDG accumulation over time in the left upper quadrant are shown indicating an inflammatory bowel segment (arrow; courtesy of [28])
In a small case study, the effectiveness of the recombinant anti-TNF antibody infliximab was analyzed [28]. Among 21 patients with CD who were treated with infliximab, 11 patients had an inflammatory stenosis, 7 of which were located in the terminal ileum and 4 in the colon. All stenoses could be verified by hydro-MRI, while four stenoses were missed by endoscopy. Of 11 patients, 9 responded well to infliximab and became symptom free after only one infusion with remissions of several months up to 18 months. One patient underwent ileocecal resection 2 weeks after infliximab treatment because of perforation with abscess formation. Further analysis of the patient histories revealed that most of the patients were concomitantly treated with immunosuppressive drugs, which may explain the overall good response to infliximab in this small case series. Infliximab may thus represent an option for the rapid medical treatment of symptomatic stenoses following careful diagnostic assessment of the nature of the stenosis.
Fistulas in Crohn’s disease
Fistulas represent a predominant chronic complication of CD and the lifelong risk of these patients developing fistulas ranges from 20 to 40% [2, 3]. Development of fistulas is facilitated by the transmural inflammatory affection of the bowel wall and indicates that the inflammation has penetrated into adjacent organs, tissue, or skin. Fistulas are classified according to their location and the connection with contiguous organs. Internal fistulas terminate into adjacent organs (e.g., enteroenteric, enterovesical, ileocolic, gastrocolic, or rectovaginal) or into the nearby mesentery. External fistulas terminate on the body surface (e.g., enterocutaneous, parastomal, or perianal). While external fistulas are often associated with the presence of local pain, drainage, and the risk of abscess formation, internal fistulas often remain unrecognized and are frequently asymptomatic. This is often the case with ileoileal or ileocecal fistulas, which rarely require intervention. Major internal fistulas such as gastrocolic fistulas, which may bypass major segments of the intestinal tract and thus cause a functional short bowel syndrome, must be removed.
Since the treatment of fistulas depends on location, severity of symptoms, number, the history of previous local surgical procedures and sphincter function, there is no general standard treatment. The optimal management of patients with fistulas requires appropriate integration of medical and surgical approaches. With regard to medical therapy, only a few studies have focused on the efficacy in healing the fistulas in CD specifically and, to date, there has been no well-accepted fistula disease activity index for the assessment of treatment response. Aminosalicylates are ineffective and corticosteroids seem to be detrimental in fistulizing CD [29, 30]. Better data exist for antibiotic therapy with metronidazole and ciprofloxacin. Several uncontrolled studies exist for metronidazole in fistulizing perianal CD [31–33]. However, the recurrence rate after discontinuation of metronidazole is up to 78% within 4 months and long-term administration of metronidazole is associated with an unacceptably high risk of neurotoxicity and paresthesias. In addition, the efficacy of ciprofloxacin has been reported in some small uncontrolled studies [34].
In one landmark study on the efficacy in CD of 6-mercaptopurine, a key metabolite of the immunosuppressive drug azathioprine, the responsiveness of fistulas was analyzed separately showing response rates of 55 vs. 24% in the placebo group [35]. Other uncontrolled trials showed similar results [36, 37]. Of note was that the mean time to response was over 3 months and up to 8 months, suggesting a delayed mechanism of action. For other immunosuppressants including methotrexate, cyclosporin A, tacrolimus, and mycophenolate mofetil, reports on their efficacy in fistulizing CD are either anecdotal or refer to small subgroup analysis only.
Anti-TNF strategies in fistulizing Crohn’s disease
Considerable advances in the medical treatment of fistulas in CD have been achieved by the development of anti-TNF strategies. The randomized, double-blind, placebo-controlled study of Present [38] focused on Crohn’s patients with enterocutaneous fistulas. A total of 94 patients with either draining abdominal fistulas (10% of patients) or perianal fistulas (90% of patients) were treated with 5 mg/kg body weight infliximab, 10 mg/kg body weight infliximab or with placebo at weeks 0, 2, and 6. Of the patients, 68% receiving 5 mg/kg infliximab and 56% receiving 10 mg/kg infliximab achieved a closure of 50% or more of open draining fistulas versus 26% of the patients receiving placebo (p=0.002, P=0.02). In addition, 55% of the patients treated with 5 mg/kg and 38% of the patients treated with 10 mg/kg showed a closure of all draining fistulas versus 13% of the placebo group. However, the mean duration of closure of draining fistulas was only about 3 months.
A very recent follow-up study (ACCENT II) addressed the question of whether repeated administration every 8 weeks in fistulizing CD can sustain an initial response achieved by infliximab [39]. After 54 weeks, 36% of patients treated repeatedly with infliximab still had complete absence of draining fistulas versus 19% treated with a placebo. Studies with CDP-571, another “humanized” anti-TNF antibody revealed response rates with more than 50% closure of draining fistulas in 50% of the CDP-571-treated patients versus 15% in the placebo group [40, 41]. The high relapse rate after anti-TNF treatment can be explained by the fact that most fistula tracts persist morphologically despite clinical remission, as could be shown in several follow-up studies by MRI and endosonography [42–44].
Given the clinical response rates, these data have raised the question of whether anti-TNF treatment could be a true alternative treatment option to surgical intervention. However, a stratification of the response rates based on the type of fistulas has not been carried out in any of the studies. Since the results of surgical interventions vary depending on the type of fistula, even among the perianal fistulas, a comparison of these interventions with anti-TNF treatment is almost impossible. Many perianal fistulas in CD, especially when the rectum is not involved, are simple and superficial, i.e., the anal sphincter lies above the fistula tract (superficial, low transsphincteric, and low intersphincteric fistulas). Most of these fistulas can be cured definitely by fistulotomy with healing rates between 70 and 100%, low recurrence rates of <20%, and low risk for incontinence [45–49]. It thus appears that in simple fistulas without rectal Crohn’s involvement, surgical treatment in combination with antibiotics is still the treatment of choice.
Complex fistulas include fistulas with many openings, openings proximal to the dentate line, fistulas with tracts, high blind extensions and high suprasphincteric or extrasphincteric fistulas. Complex fistulas should be analyzed by MRI and examined under anesthesia to define disease extent and to identify abscesses that require unroofing and drainage. In these patients, surgical fistulotomy may be associated with significant morbidity [50–52]. However, it is generally felt that surgical treatment should be combined with medical treatment in these patients, whenever possible. There are no studies comparing surgical treatment with a combination of surgical treatment and conventional medical treatment, which have included antibiotics and immunosuppression with azathioprine or 6-mercaptopurine respectively. A small recent study in 32 patients with perianal fistulizing CD has compared the efficacy of infliximab alone with infliximab as an adjunct to examination under anesthesia (EUA) and with seton placement [53]. Those patients who had a EUA prior to infliximab had an initial response of 100 vs. 82% in those patients who received infliximab only (p=0.014). The recurrence rate was 44 vs. 79% (p=0.001) and the time to recurrence was longer (13.5 months vs. 3.6 months, p=0.0001). Findings from a small retrospective case series in which a single center experience of 29 patients was reviewed support the notion that combination of seton placement, infliximab infusion and immunosuppression is beneficial in complicated perianal and rectovaginal fistulas [54].
In another retrospective study, the question was addressed as to what proportion patients treated with infliximab eventually undergo surgery [55]. It was found that 6 out of 26 patients had complete fistula closure and 12 had a partial response to infliximab. Out of 26 patients, 14 still required surgery (10 bowel resection, 4 perianal procedures); however, an additional 6 patients with persisting draining fistulas declined surgery.
Even fewer data exist on the medical treatment of internal fistulas. Fistulas involving the upper GI tract may cause severe functional short bowel syndrome, while ileosigmoid fistulas may induce severe diarrhea, both due to a by-pass effect. These types of fistula need to be treated. A follow-up study on 24 patients with internal fistulas evaluated the effect of nonoperative therapy. Ten patients required surgery within 1 year, another 8 patients within 9 years [56]. These observations date from the pre-immunosuppression and pre-anti-TNF eras. An attempt of medical treatment might thus be appropriate in patients with mild or moderately symptomatic enteroenteric fistulas.
Rectovaginal fistulas occur in about 9% of women with anal CD [57, 58]. Symptoms include passage of stool and flatus from the vagina, drainage of purulent material, yeast infections, perineal pain or dyspareunia, and can range from minimal to incapacitating. Surgical interventions include insertion of a seton or mushroom drain for drainage, fistulotomy of low anovaginal fistulas, and transrectal, transvaginal or cutaneous advancement flaps with temporary proximal bowel diversion. Initial success rates are high, between 70 and 75%, but recurrence rates tend to be higher than in patients with anorectal fistulas [57, 59–61].
Data on medical therapy for rectovaginal fistulas result from a few small case series. In 2 out of 6 patients with rectovaginal fistulas and 1 out of 2 patients with vulvar fistulas, 6-mercaptopurine led to complete fistula closure. Two patients responded with partial closure [36]. In 5 patients with a total of 12 fistulas including 5 enterovaginal fistulas, intravenous cyclosporin A led to rapid improvement within several days [62]. To date there have been no prospective studies on the treatment of rectovaginal fistulas with anti-TNF strategies.
Enterovesical fistulas can be found in about 6% of patients with IBD. There is controversy as to whether enterovesical fistulas represent an indication for surgery per se or whether medical treatment can be attempted. In a recent case report, a 31-year-old patient with ileovesical fistulas was treated successfully with infliximab [63].
Conclusion
The management of fistulizing CD requires the close interdisciplinary collaboration of the gastroenterologist and the surgeon. The general principles in the management of fistulas in CD can be defined as follows:
-
1.
Proper mapping of the fistulas, if necessary by examination under anesthesia and MRI
-
2.
Draining of abscesses
-
3.
Eradication of the fistula tract by medical and/or surgical treatment
-
4.
Prevention of recurrence
-
5.
Preservation of continence and sphincter integrity
Considering the high success rates and low recurrence rates, simple and low-lying fistulas should still be treated surgically. Medical treatment can be attempted first in cases of complex fistulas, especially when sphincter integrity may be impacted. Here, the novel anti-TNF strategies are valuable treatment options. Clinical response rates have increased significantly, especially on a short-term basis, and infliximab has proved effective in placebo-controlled trials for reducing the number of draining fistulas and maintenance of that reduction. However, so far, the availability of anti-TNF treatment has not completely redefined the border between medical and surgical therapy. Furthermore, concomitant immunosuppressive therapy is required to prevent the formation of human anti-chimeric antibodies (HACA), which may limit the efficacy of anti-TNF antibodies upon repeated administration, and allergic reactions and serious infections may occur [64, 65]. In this context, infections such as bronchitis and pneumonia, as well as reactivation of tuberculosis, have been described after anti-TNF therapy. In addition, the potential risk of the development of cancer after anti-TNF therapy remains unclear. Therefore, clinical therapy of CD with anti-TNF antibodies always requires strict calculation of potential benefits and risks for the patient.
Prospective trials on medical therapy and the combination of medical and surgical therapy for complex fistulas and internal fistulas are needed to define the potential and the limitations of these novel therapeutic approaches.
References
Shivananda S et al (1996) Incidence of inflammatory bowel disease across Europe. Is there a difference between North and South. Gut 39:690–697
Farmer RG, Hawk WA, Turnbull RBJ (1975) National cooperative Crohn’s disease study: extraintestinal manifestations and perianal complications. Gastroenterology 77:914–920
Steinberg DM, Cooke WT, Alexander-Williams J (1973) Abscess and fistulae in Crohn’s disease. Gut 14:865–869
Holtmann M et al (2002) Das mukosale Immunsystem. Wie klar ist die Pathogenese. Internist 43:1343–1353
Hugot JP et al (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603
Ogura Y et al (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606
Neurath MF, Finotto S, Glimcher LH (2002) The role of Th1/Th2 polarization in mucosal immunity. Nat Med 8:567–573
Holtmann M et al (2002) Functional relevance of soluble and transmembrane TNF and TNF-signal transduction in gastrointestinal diseases with special reference to inflammatory bowel diseases. Z Gastroenterol 40:587–600
Siegel SA et al (1995) The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine 7:15–25
Knight DM et al (1993) Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol 30:1443–1453
Scallon BJ et al (1995) Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 7:251–259
Luegering A et al (2001) Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 121:1145–1157
Ewe K et al (1993) Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn’s disease. Gastroenterology 105:367–372
Candy S et al (1995) A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut 37:674–678
Sandborn WJ, Sutherland L, Pearson DC, May GR, Modigliani R, Prantera C (1999) Azathioprine or 6-mercaptopurine for inducing remission of Crohn’s disease. The Cochrane Library. Update Software, Oxford
Pearson DC et al (2000) Azathioprine for maintaining remission of Crohn’s disease. Cochrane Database Syst Rev 2(Ia):CD000067
Tiede I et al (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 111:1133–1145
Neurath MF, Stange E (2000) Evidenzbasierte Immunsuppression bei chronisch entzündlichen Darmerkrankungen. Dtsch Ärztebl 97:A1977
Fazio VW et al (1989) Stricture-plasty in Crohn’s disease. Ann Surg 210:621–625
Fazio VW et al (1996) Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. Ann Surg 224:563–573
Van Dullemen HM et al (1995) Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 109:129–135
Targan SR et al (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 337:1029–1035
Schunk K et al (2000) Hydro-MRI in Crohn’s disease: appraisal of disease activity. Invest Radiol 35:431–437
Schunk K et al (2000) Assessment of inflammatory activity in Crohn disease with hydro-MRI. Röfo Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr 172:153–160
Bartenstein P et al (1997) Central motor processing in Huntington’s disease. A PET study. Brain 120:1553–1567
Bicik I et al (1997) Inflammatory bowel disease activity measured by positron-emission tomography. Lancet 350:262
Neurath MF et al (2002) Non-invasive assessment of Crohn’s disease activity: a comparison of 18F-fluoro-deoxy-glucose-positron emission tomography. Hydromagnetic resonance imaging and granulocyte scintigraphy with labelled antibodies. Am J Gastroenterol 97:1878–1885
Holtmann M et al (2003) Anti-TNF antibodies in the treatment of inflammatory intestinal stenoses in Crohn’s disease. Z Gastroenterol 41:11–17
Jones JH, Lennard-Jones JF (1966) Corticosteroids and corticotropin in the treatment of Crohn’s disease. Gut 7:181–187
Malchow H et al (1984) European cooperative Crohn’s disease study (ECCDS): results of drug treatment. Gastroenterology 86:249–266
Brandt LJ et al (1982) Metronidazole therapy for perineal Crohn’s disease: a follow-up study. Gastroenterology 83:383–387
Bernstein LH et al (1980) Healing of perineal Crohn’s disease with metronidazole. Gastroenterology 79:357–365
Schneider MU et al (1985) Metronidazol in der Behandlung des Morbus Crohn. Dtsch Med Wochenschr 110:1724–1730
Turunen U, Farkkila M, Seppala K (1989) Long-term treatment of perianal or fistulous Crohn’s disease with ciprofloxacin. Scand J Gastroenterol 24:144
Present DH et al (1980) Treatment of Crohn’s disease with 6-mercaptopurine: a long-term randomized double blind study. N Engl J Med 302:981–987
Korelitz BI, Present DH (1985) Favorable effect of 6-mercaptopuine on fistulae of Crohn’s disease. Dig Dis Sci 30:58–64
O’Brien JJ, Bayless TM, Bayless JA (1991) Use of azathioprine or 6-mercaptopurine in the treatment of Crohn’s disease. Gastroenterology 101:39–46
Present DH et al (1999) Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 340:1398–1405
Sands BE et al (2004) Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 350:934–936
Sandborn WJ et al (2001) An engineered human antibody to TNF (CDP571) for active Crohn’s disease: a randomized, double-blind placebo-controlled trial. Gastroenterology 120:1330–1338
Feagan BG et al (2000) A randomized, double-blind, placebo-controlled, multi-center trial of the engineered human antibody to TNF (CDP571) for steroid sparing and maintenance of remission in patients with steroid-dependent Crohn’s disease [abstract]. Gastroenterology 118:A655
Bell SJ et al (2003) Response of fistulating Crohn’s disease to infliximab treatment assessed by magnetic resonance imaging. Aliment Pharmacol Ther 17:387–393
Van Bodegraven AA et al (2002) Endosonographic evidence of persistence of Crohn’s disease-associated fistulas after infliximab treatment, irrespective of clinical response. Dis Colon Rectum 45:39–45
Rasul I et al (2004) Clinical and radiological responses after infliximab treatment for perianal fistulizing Crohn’s disease. Am J Gastroenterol 99:82–88
Marks CG, Ritchie JK, Lockhart-Mummery HE (1981) Anal fistulas in Crohn’s disease. Br J Surg 68:525–527
Bernard D, Morgan S, Tasse D (1986) Selective surgical management of Crohn’s disease of the anus. Can J Surg 29:318–321
Williamson P et al (1995) Twenty year review of the surgical management of perianal Crohn’s disease. Dis Colon Rectum 38:389–392
Nordgren S, Fasth S, Hulten L (1992) Anal fistulas in Crohn’s disease: incidence and outcome of surgical treatment. Int J Colorectal Dis 7:214–218
Williams J et al (1991) Fistula-in-ano in Crohn’s disease. Dis Colon Rectum 34:378–384
White R et al (1990) Seton management of complex anorectal fistulas in patients with Crohn’s disease. Dis Colon Rectum 33:587–589
Pearl RK et al (1993) Role of the seton in the management of anorectal fistulas. Dis Colon Rectum 39:573–577
Scott H, Northover J (1996) Evaluation of surgery for perianal Crohn’s fistulas. Dis Colon Rectum 39:1039–1043
Regueiro M, Mardini H (2003) Treatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placement. Inflamm Bowel Dis 9:98–103
Topstad DR et al (2003) Combined seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizing anorectal Crohn’s disease: a single center experience. Dis Colon Rectum 46:577–583
Poritz LS, Rowe WA, Koltun WA (2002) Remicade does not abolish the need for surgery in fistulizing Crohn’s disease. Dis Colon Rectum 45:771–775
Broe PO, Bayless TM, Cameron JL (1982) Crohn’s disease: are enteroenteral fistulas an indication for surgery? Surgery 91:249–253
Radcliffe A et al (1988) Anavaginal and rectovaginal fistulas in Crohn’s disease. Dis Colon Rectum 31:94–99
Scott N, Nair A, Hughes L (1992) Anorectal and rectovaginal fistula with Crohn’s disease. Br J Surg 79:1379–1380
Joo JS et al (1998) Endorectal advancement flap in perianal Crohn’s disease. Am Surg 64:147–150
Hesterberg R et al (1993) Treatment of anovaginal fistulas with an anocutaneous flap in patients with Crohn’s disease. Int J Colorectal Dis 1993:51–54
Ozuner G et al (1996) Long-term analysis of the use of transanal rectal advancement flaps for complicated anorectal/vaginal fistulas. Dis Colon Rectum 39:10–14
Hanauer SB, Smith MB (1993) Rapid closure of Crohn’s disease fistulas with continuous intravenous cyclosporin. Am J Gastroenterol 88:646–649
Game X et al (2003) Infliximab treatment of Crohn disease ileovesical fistula. Scand J Gastroenterol 38:1097–1098
Beart F et al (2003) Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 348:601–608
Keane J et al (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345:1098–1104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holtmann, M.H., Neurath, M.F. Anti-TNF strategies in stenosing and fistulizing Crohn’s disease. Int J Colorectal Dis 20, 1–8 (2005). https://doi.org/10.1007/s00384-004-0634-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-004-0634-0