Abstract
Background and aims
Evaluation of cytokeratin 20 (CK20) specific quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) and immunohistochemistry (IHC) for detection of occult tumor cells in lymph nodes of 72 patients with colorectal carcinoma (UICC stage I and II).
Methods
Serial sections of formalin-fixed, paraffin-embedded lymph nodes (mean 14.3/case) were used for microdissection, RNA isolation and QRT-PCR and for CK20 IHC using routine protocols. Results of QRT-PCR and IHC were compared and correlated to the CK20 expression pattern of the primary tumors and clinical follow-up.
Results
IHC revealed CK20-positive tumor cells in lymph nodes of 14.5% (10/69) and 0% (0/3) cases with a CK20-positive and CK20-negative primary tumor, respectively. CK20 mRNA was detected in the lymph nodes of 36.8% (7/19) cases by QRT-PCR with all 7 cases also expressing CK20 mRNA in the primary tumor. CK20 mRNA (QRT-PCR) and protein (IHC) detection in serial sections did not agree in 25% (5/20) of cases. A trend was seen towards a worse disease course for patients with CK20-positive lymph nodes by IHC (incidence of recurrent disease) and QRT-PCR (disease-free survival, incidence of recurrent disease).
Conclusion
CK20-specific IHC and QRT-PCR are supportive tools to conventional histology for detection of occult tumor cells in archival tissues, with the restriction that a laborious QRT-PCR procedure is necessary to achieve appropriate specificity. A prognostic value of CK20 IHC or QRT-PCR for stratification of UICC stage I and II patients into those likely to develop recurrent disease was not evident.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In colorectal cancer (CRC), histopathological lymph node status at the time of resection of the primary tumor is one of the main prognostic factors. As such, patients with lymph node involvement (N+; UICC III/Dukes' C, 5-year survival 30–55%) generally have a worse prognosis than patients without lymph node involvement (N0; UICC I, II/Dukes' A, B, 5-year survival 60–95%) and benefit from adjuvant therapy [1, 2]. The identification of metastasis in regional lymph nodes is therefore important for precise staging and further treatment strategy [3]. This is achieved by routine histological assessment using random hematoxylin and eosin (H&E) stained sections of lymph nodes excised together with the primary tumor. A potential improvement of this approach is represented by the concept of "sentinel" lymph node examination [4]. Despite the well accepted use of H&E staining for lymph node staging, the presence of very few or single tumor cells may escape even a most experienced investigator. However, whether these occult tumor cells or "micrometastasis" are responsible for recurrent disease, which develops in about 20% of "node-negative" CRC cases, is controversial and a clearcut association of micrometastasis and (poor) prognosis has not been shown [5, 6, 7, 8].
As precise staging is required for optimal patient management, novel techniques for the detection of disseminated tumor cells in lymph nodes as well as in blood and bone marrow have been evaluated in recent years [9, 10, 11]. In order to represent an acceptable alternative to the current gold standard of routine histopathology, novel techniques should provide higher specificity and sensitivity for the detection of disseminated tumor cells. Whereas specificity is defined by tumor specific [12] or more frequently tissue or cell type specific markers [13, 14, 15], sensitivity is dependent on the assay type [immunohistochemistry (IHC) or polymerase chain reaction (PCR)] and protocol [16]. Few markers and assays reach both 100% specificity and sensitivity, causing "false negative" and/or "false positive" results especially when using molecular techniques [17]. Moreover, identification of a "positive" sample does not necessarily implicate the presence of "active" tumor cells but may also reflect the presence of "shed" cellular material or DNA [6]. Therefore the specificity and sensitivity of novel assays varies between laboratories and the prognostic impact remains debatable [8].
In CRC cytokeratin 20 (CK20) has been suggested as a promising marker for the detection of disseminated tumor cells due to its restricted expression pattern [18, 19] and the apparent lack of any pseudogenes [20]. Moreover, CK20 was shown to be expressed in the majority of colorectal tumors [21, 22] and hence several studies applied CK20-specific assays to detect disseminated CRC cells in blood [23, 24, 25, 26], lymph nodes [27, 28, 29, 30], and bone marrow [31, 32] of CRC patients. However, the prognostic value of CK20-positive "micrometastasis" in lymph nodes of CRC patients has been evaluated only in few studies [28, 33]. Moreover, the specificity and sensitivity of CK20 as marker for disseminated tumor cells remains controversial [34, 35, 36, 37]. This may be explained by two points. First, the development of CK20-specific assays was based on observations of a very restricted CK20 protein expression pattern (high specificity). However, only few studies have used pan-cytokeratin [38, 39] or CK20 [31] specific antibodies for detection of CK20 protein expressing, disseminated CRC cells. In contrast, most assays are based on detection of CK20 mRNA (high sensitivity) and share the problem of a reduced specificity due to CK20 mRNA expression in nonepithelial cells [34, 35, 36]. This limitation is especially apparent when tissue acquisition is not checked morphologically. Second, both CK20 mRNA and protein expression may be very heterogeneous in primary colorectal tumors [40], and therefore the value of CK20-specific assays for the detection of disseminated CRC cells must be considered together with the CK20 expression pattern of each individual primary tumor.
Here we examined formalin-fixed and paraffin-embedded lymph nodes of 72 UICC stage I and II (T1–4, N0, M0) CRC patients for the presence of CK20-positive tumor cells by both IHC (n=72) and quantitative reverse transcriptase (QRT) PCR (n=20). The use of serial sections for IHC and QRT-PCR analysis allowed a direct comparison of CK20 mRNA and protein expression and the specificity/sensitivity of the two approaches. Finally, we correlated of the CK20 expression pattern observed in the lymph nodes to that of the corresponding primary tumor and to the clinical follow-up findings.
Materials and methods
Patients and tissues
This study included 72 patients with CRC (T1–4, N0, Mx or M0, according to WHO classification 2000) who had received complete resection of their primary tumor and lymph nodes between 1994 and 1996. Clinical follow-up data were obtained from all cases (median 68 months). Both the resected primary tumor and lymph nodes (mean 14.3±8 lymph nodes per case) were available as formalin-fixed, paraffin-embedded specimens from all cases (archive, Institut für Pathologie, Technische Universität München, Munich, Germany). Analysis of CK20 protein and mRNA expression of primary tumors has been reported for part of this patient group [40]. For each patient three serial, 5-µm tissue sections were cut from the paraffin blocks containing the resected lymph nodes (each block containing one to six lymph nodes, thus more than 1 paraffin block per case). These were then used for either (a) staining with H&E, (b) CK20 IHC, or (c) microdissection and RNA isolation, as shown in Fig. 1 and described below. For the entire study approx. 1044 (72 patients) and 290 (20 patients) lymph nodes were analyzed by CK20-specific IHC and QRT-PCR, respectively.
Flowchart showing the procedures of CK20 mRNA and protein expression analysis in "node-negative" lymph nodes (LN) of colorectal cancer patients. For each case all resected lymph nodes (mean 14.3±8; embedded in one to six tissue blocks, with each one to six lymph nodes) were analyzed (see text), as shown in the upper left as example: one case with 14 lymph nodes in three tissue blocks 1a–1c. IHC Immunohistochemistry, QRT-PCR quantitative reverse transcriptase polymerase chain reaction
Immunohistochemistry
H&E staining and CK20 IHC were performed according to routine protocols. For CK20 IHC citrate-buffered antigen retrieval in a pressure cooker was followed by incubation with the CK20 mouse monoclonal antibody IT-Ks 20.8 (Progen, Heidelberg, Germany) and detection by a labeled strepavidin-biotin/alkaline-phosphatase complex (Dako, Hamburg, Germany). Epithelial cells of the normal colonic mucosa served as internal positive control. CK20 IHC in primary tumor specimens was scored essentially as described previously [40], with "−" indicating no CK20-positive tumor cell. "+/−" 10–90% CK20-positive tumor cells (heterogeneous), and "+" more than 90% of CK20-positive tumor cells. For scoring CK20 IHC of lymph nodes the following semiquantitative system was used: "−" all lymph nodes (per case) negative, including lymph nodes with unspecific CK20 staining outside the LN capsule; "+" at least one lymph node (per case) with one or more CK20-positive stained tumor cells within the lymph node capsule.
Microdissection and RNA isolation
Tissue sections were treated with xylene and graded alcohols (100%–50%), individually stained with instant hematoxylin (Shandon, Frankfurt, Germany), and lymph node cells were dissected from within the lymph node capsule using fine needles (Fig. 1). For each case all lymph nodes were dissected separately from two to six sections (each one to six lymph nodes) per case, but cell preparations were then pooled in one Eppendorf tube (i.e., one sample per case, containing the pooled cells dissected from all resected lymph nodes of that case). From this, RNA was isolated as described previously [40]: Briefly, cells were immediately lysed in 500 µl digestion buffer (10 mM Tris-HCl, 0.1 mM EDTA, 2% sodium dodecyl sulfate, and 0.5 mg proteinase K; all from Sigma, Taufkirchen, Germany) and incubated overnight at 60°C, 350–400 rpm. This was followed by phenol:choroform extraction, precipitation of nucleic acids in isopropanol, and a DNase digestion step. For this, 10 U DNase I (Roche Diagnostics, Mannheim, Germany), 20 µl DNase buffer (0.4 M Tris-HCl, 60 mM MgCl2, 0.1 M NaCl), and up to 200 µl H2O were incubated with the RNA pellet for 45 min at 37°C. Thereafter RNA was reextracted by phenol:chloroform extraction, precipitation as above, and resuspension in H2O. A 1:100 dilution of RNA extracts was measured in a conventional spectrophotometer (DU530, Beckman, Fullerton, USA), determining absorbance at 260 and 280 nm (A260/280).
Synthesis of cDNA and PCR
RNA (500 ng) was reverse-transcribed into cDNA and processed for quantitative PCR using the CK20 LC mRNA quantification kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the supplied protocols. The design of the two fluorescent hybridization probes and primers ensures amplification and measurement of signals obtained from exclusively cDNA. Two negative controls (for RT and PCR reactions) and one positive control ("calibrator," included in the kit) were analyzed together with 13 study samples. Both CK20 and porphobilinogen deaminase (PBGD, reference gene) gene expression was determined for each sample in a single run. LightCycler® data was analyzed by the relative quantification software (Roche Diagnostics GmbH, Mannheim, Germany). This expresses CK20 gene levels as a relative ratio (CK20:PBGDsample) divided by (CK20:PBGDcalibrator), thereby correcting both for sample loading and PCR efficiency.
The sensitivity of this CK20-specific QRT-PCR approach has been determined previously by cell dilution experiments [40], with a detection limit of 1 CK20-positive cell in 100,000 CK20-negative cells (three of four experiments) for formalin-fixed and paraffin-embedded samples.
Results
Immunohistochemical detection of CK20-positive cells in "node negative" lymph nodes
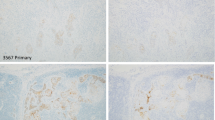
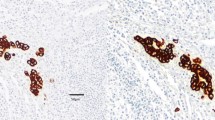
Lymph nodes from a total of 72 CRC patients (mean 14.3±8 per case, 1044 lymph nodes in total), staged as "node negative" by conventional H&E staining, were analyzed by immunohistochemistry for CK20 protein expressing cells. Corresponding primary tumors had either been studied before [40] or were analyzed in parallel. Two major patterns of CK20 immunoreactivity were observed in these lymph nodes (Fig. 2): (a) undetectable/negative staining of the entire lymph node (score −) and (b) a single or several positively stained tumor cell(s) within the lymph node capsule (score +). In contrast, lymph nodes of CRC patients with prominent lymph node metastasis exhibited strong CK20-specific signals in the infiltrating tumor cells. A case was scored "IHC-positive" if one or more of the resected lymph nodes contained CK20-positive tumor cells (see above).
Upon correlation of these lymph node CK20 staining patterns with those of the corresponding primary tumor, the following observations were made (Table 1). None of the three cases with a CK20-negative primary tumor had lymph nodes with CK20-expressing tumor cells. For those cases with a CK20-positive primary tumor, CK20-positive tumor cells were detected in the lymph nodes of 10/69 (14.5%) cases, whereby two and eight of the ten cases derived from primary tumors with a heterogeneous and strongly positive CK20 expression pattern, respectively.
Finally, all cases were divided into the two groups of "IHC-negative" (62/72) and IHC-positive" (10/72, one or more lymph nodes with CK20-positive tumor cells). The results were then correlated to histopathological characteristics of the primary tumor (location, differentiation grade, and T category). As shown in Table 2, no significant differences were seen between the two groups, with an equal location of the primary tumor and a predominant differentiation grade of 2 and T category 3. Moreover, there was no association between the number of resected lymph nodes and CK20-specific tumor cell detection by IHC (data not shown).
Detection of CK20 mRNA in "node-negative" lymph nodes
To extend the analysis of CK20 expression in resected primary tumors and corresponding lymph nodes from the protein to the mRNA level, we examined CK20 mRNA expression by QRT-PCR in primary tumors [40] and lymph nodes in 20 of 72 cases (290 lymph nodes in total). Microdissection of exclusively cells lying within the lymph node capsule prevented "false-positive" results from contaminating cells (for example, "carry over" from surgery) or debris from outside the lymph node tissue. To achieve this all lymph nodes per case (from two to six paraffin blocks, with each one to six lymph nodes) were microdissected separately, but then pooled for further analysis (see above; Fig. 1). As summarized in Table 3, no CK20 mRNA was detected in the lymph nodes of a single case which did not express CK20 mRNA in the primary tumor. Of the remaining 19 cases with detectable CK20 mRNA expression in the primary tumor 36.8% (7/19) and 63.2% (12/19) cases had positive and negative CK20 mRNA expression in lymph nodes, respectively. Again, detection of CK20-expressing cells was not correlated with the number of lymph nodes resected/investigated (data not shown).
Correlation of CK20 QRT-PCR and IHC positivity
Despite a good correlation of CK20 gene and protein expression in primary tumors [40], we again compared CK20 gene and protein expression in those cases for which lymph node samples had been analyzed by both CK20 IHC and QRT-PCR on serial sections (n=20; Table 4, Fig. 3). Of 13 cases (65%) with undetectable CK20 protein expression by IHC, 8/13 (61.5%) were negative and 5/13 (38.5%) positive for CK20 mRNA expression by QRT-PCR. In contrast, of the 7/20 cases (35%) with a positive IHC result, 2/7 (28.6%) were also positive by QRT-PCR and 5/7 (71.4%) did not yield a positive QRT-PCR result.
Comparison of CK20 mRNA and protein expression in "node-negative" lymph nodes. CK20 mRNA expression (relative ratio ×106, as described in the text) was correlated to cases with CK20-negative (n=13) and CK20-positive (n=7) stained lymph nodes (see also Table 4)
Finally, in cases with positive CK20-specific QRT-PCR signals in lymph nodes (7/20) the levels of CK20 mRNA were distributed over a wide range (relative ratio: 0,034–0,78×106), in both the IHC negative and positive subgroups (Fig. 3). However, there appeared to be two clusters, with very high (above 0.4×106) and low (below 0.2×106) CK20 mRNA expression. For comparison, "node-positive" lymph nodes (N1–2) processed in the same way as in this study had a mean CK20 mRNA level of 0.46×106 [40], or ranged from about 1×103 to 1×106 when entire lymph nodes were analyzed [37].
Correlation of CK20 IHC and QRT-PCR to clinical follow-up results
To determine whether the detection of occult, CK20-positive tumor cells in lymph nodes of CRC patients by either IHC or QRT-PCR identifies patients with a worse prognosis and/or a predisposition to develop metastasis, we examined the correlation between IHC (n=72) and QRT-PCR (n=20) findings to the clinical follow-up (statistical analysis was omitted due to low case numbers). A trend towards a worse disease course was observed in cases with a positive CK20 result by IHC (Table 5) and QRT-PCR (not shown). The first observation was a shorter median disease-free survival in cases with a positive (43 months) than a negative (68 months) QRT-PCR result. Moreover, the incidence of recurrent disease was higher for IHC (20%) and QRT-PCR (57.1%) positive cases than for IHC (14.5%) and QRT-PCR (38.5%) negative cases. Finally, overall survival was shorter in QRT-PCR positive (64 months) than negative (68 months) cases.
Discussion
CK20 has been suggested as a potentially useful marker of disseminated tumor cells in CRC due to its restricted expression pattern [18, 19]. While IHC has been used to characterize CK20 protein expression in primary tumors and overt metastases, RT-PCR has been the method of choice to detect occult tumor cells in bone marrow [31], blood [23, 24, 25, 26], and lymph nodes [27, 28, 30] of CRC patients. Despite these studies the value of CK20-specific RT-PCR for detection of disseminated tumor cells and its potential prognostic impact remain an open question. In fact, this may be due to the use of IHC to characterize CK20 protein expression in primary tumors [21, 22] and using this expression pattern directly as basis for CK20 mRNA detection of disseminated cells. However, CK20 protein levels may not directly reflect mRNA levels. Moreover, primary tumors are very heterogeneous and may differ in the CK20 expression profile of tumor cells [40]. Thus, not every tumor cell disseminated from a heterogeneous primary tumor expresses CK20 protein or mRNA.
The present study therefore evaluated in detail the value of CK20 as marker for the detection of occult tumor cells in "node-negative" lymph nodes of CRC patients. Moreover, the prognostic impact of CK20-positive tumor cells in "node-negative" lymph nodes was investigated. For this, CK20 mRNA and protein expression was analyzed in lymph nodes and in the corresponding primary tumor of CRC patients. Morphologically controlled tissue acquisition of lymph node cells within the lymph node capsule combined with quantitative CK20-specific QRT-PCR and the analysis of CK20 protein expression by IHC on a serial section, as first described in the present study, ensured specific and sensitive measurements.
Whereas none of the cases with a CK20 mRNA and protein negative primary tumor had any positive signals in the lymphnodes (IHC and QRT-PCR), both CK20-specific IHC and QRT-PCR revealed CK20 protein expressing tumor cells and CK20 mRNA in "node-negative" lymph nodes in patients with a CK20-positive primary tumor (IHC 14.5% and QRT-PCR 36.8%). In comparison to other studies [27, 28, 32, 33, 41], we detected CK20-positive tumors cells in a smaller group of cases, potentially indicating a higher specificity of our approach. In particular, the possibility of measuring tumor cell "carry-over" due to surgery can be ruled out in our approach, as we specifically analyzed cells within the lymph node capsule.
However, there was a discrepancy between the CK20-positive cases identified by CK20-specific IHC and QRT-PCR. For example, QRT-PCR detected seven cases with CK20-positive lymph nodes, but only three of these were also positive by IHC. The "false-negative" results in the other four cases may reflect the fact that CK20 mRNA was not translated into protein and was therefore not detected by the CK20-specific antibody. In contrast, of five cases in which CK20-positive tumor cells were identified in lymph nodes by IHC only two showed a CK20-positive signal by QRT-PCR. Although we used serial sections for IHC and QRT-PCR analysis, we cannot entirely rule out that single tumor cells present in the IHC section were lost on the following QRT-PCR dedicated section.
With respect to the prognostic value of CK20-specific IHC and QRT-PCR for detection of disseminated tumor cells in lymph nodes of CRC patients, cases with a positive CK20 reaction in lymph nodes appeared to have a higher incidence of recurrent disease (IHC 20% and QRT-PCR 57.1%) than those cases with CK20-negative lymph nodes (IHC 14.5% and QRT-PCR 38.5%). Only in cases with detectable CK20 mRNA (QRT-PCR) in lymph nodes was this also reflected in a shorter disease-free and overall survival times. Due to the low number of cases with CK20-positive lymph nodes by both IHC and QRT-PCR the prognostic value of combined IHC and QRT-PCR could not be determined. Nevertheless, the data suggest a possible prognostic value of CK20-specific QRT-PCR. In fact, Rosenberg et al. [28] have shown that by analysis of 30 sections of two peritumoral lymph nodes by conventional CK20-specific QRT-PCR, a CK20-positive QRT-PCR signal is an independent prognostic marker in CRC patients of UICC stage I and II. Moreover, in a recent study of 141 colon cancer patients Merrie et al. [33] demonstrated a significant prognostic value of CK20 QRT-PCR for staging of CRC patients.
In summary, conventional CK20-specific IHC is suitable for the detection of tumor cells in lymph nodes of CRC patients staged as "node-negative" by routine H&E staining.CK20-specific IHC may represent a supportive tool to the conventional histopathological staging of lymph nodes in CRC patients. Similarly, CK20-specific quantitative QRT-PCR is able to identify CK20-positive lymph nodes, but the origin of these CK20 signals is unclear unless tissue acquisition and accompanying H&E or CK20-staining of serial sections is checked morphologically. Only this rather extensive sample preparation ensures an adequate balance of a high specificity and sensitivity. Finally, our present study suggests a prognostic value of CK20 IHC and/or QRT-PCR in CRC, but this must be confirmed in a larger series of patients.
References
Ragnhammar P, Hafstrom L, Nygren P et al (2001) A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol 40–:282–308
Yarbro JW, Page DL, Fielding LP et al (1999) American Joint Committee on Cancer prognostic factors consensus conference. Cancer 86:2436–2446
Cohen AM, Tremiterra S, Candela F et al (1991) Prognosis of node-positive colon cancer. Cancer 67:1859–1861
Saha S, Bilchik A, Wiese D et al (2001) Ultrastaging of colorectal cancer by sentinel lymph node mapping technique-a multicenter trial. Ann Surg Oncol 8 [9 Suppl]:94S–98S
Merrie AE, Yun K, van Rij AM et al (1999) Detection and significance of minimal residual disease in colorectal cancer. Histol Histopathol 14:561–569
Yamamoto N, Kato Y, Yanagisawa A et al (1997) Predictive value of genetic diagnosis for cancer micrometastasis: histologic and experimental appraisal. Cancer 80:1393–1398
Hermanek P (1999) Disseminated tumor cells versus micrometastasis: definitions and problems. Anticancer Res 19:2771–2774
Tsavellas G, Patel H, Allen-Mersh TG (2001) Detection and clinical significance of occult tumour cells in colorectal cancer. Br J Surg 88:1307–1320
Pantel K, von Knebel Doeberitz M (2000) Detection and clinical relevance of micrometastatic cancer cells. Curr Opin Oncol 12:95–101
Ghossein RA, Bhattacharya S, Rosai J (1999) Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res 5:1950–1960
Burchill SA, Selby PJ (2000) Molecular detection of low-level disease in patients with cancer. J Pathol 190:6–14
Sanchez-Cespedes M, Esteller M, Hibi K et al (1999) Molecular detection of neoplastic cells in lymph nodes of metastatic colorectal cancer patients predicts recurrence. Clin Cancer Res 5:2450–2454
Ghossein RA, Scher HI, Gerald WL et al (1995) Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J Clin Oncol 13:1195–1200
Hoon DS, Wang Y, Dale PS et al (1995) Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol 13:2109–2116
Lindemann F, Schlimok G, Dirschedl, P et al (1992) Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet 340 685–689
Calaluce R, Miedema BW, Yesus YW (1998) Micrometastasis in colorectal carcinoma: a review. J Surg Oncol 67:194–202
Ghossein RA, Carusone L, Bhattacharya S (1999) Review: polymerase chain reaction detection of micrometastases and circulating tumor cells: application to melanoma, prostate, and thyroid carcinomas. Diagn Mol Pathol 8:165–175
Moll R, Zimbelmann R, Goldschmidt MD et al (1993) The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation 53:75–93
Calnek D, Quaroni A (1993) Differential localization by in situ hybridization of distinct keratin mRNA species during intestinal epithelial cell development and differentiation. Differentiation 53:95–104
Burchill SA, Bradbury MF, Pittman, K et al (1995) Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer 71:278–281
Moll R, Lowe A, Laufer J et al (1992) Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 140:427–447
Wildi S, Kleeff J, Maruyama H et al (1999) Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res 5:2840–2847
Wharton RQ, Jonas SK, Glover C et al (1999) Increased detection of circulating tumor cells in the blood of colorectal carcinoma patients using two reverse transcription-PCR assays and multiple blood samples. Clin Cancer Res 5:4158–4163
Bustin SA, Gyselman VG, Williams NS et al (1999) Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer 79–:1813–1820
Wyld DK, Selby P, Perren TJ et al (1998) Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer 79:288–293
Funaki NO, Tanaka J, Ohshio G et al (1998) Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 77:1327–1332
Futamura M, Takagi Y, Koumura H et al (1998) Spread of colorectal cancer micrometastases in regional lymph nodes by reverse transcriptase-polymerase chain reactions for carcinoembryonic antigen and cytokeratin 20. J Surg Oncol 68:34–40
Rosenberg R, Hoos A, Mueller J et al (2002) Prognostic significance of cytokeratin-20 reverse transcriptase polymerase chain reaction in lymph nodes of node-negative colorectal cancer patients. J Clin Oncol 20:1049–1055
Yun K, Merrie AE, Gunn J et al (2000) Keratin 20 is a specific marker of submicroscopic lymph node metastases in colorectal cancer: validation by K-RAS mutations. J Pathol 191:21–26
Miyake Y, Yamamoto H, Fujiwara Y et al (2001) Extensive micrometastases to lymph nodes as a marker for rapid recurrence of colorectal cancer: a study of lymphatic mapping. Clin Cancer Res 7:1350–1357
Litle VR, Warren RS, Moore D 2nd et al (1997) Molecular cytogenetic analysis of cytokeratin 20-labeled cells in primary tumors and bone marrow aspirates from colorectal carcinoma patients. Cancer 79:1664–1670
Weitz J, Kienle P, Magener A et al (1999) Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res 5:1830–1836
Merrie AE, Van Rij AM, Dennett ER et al (2003) Prognostic significance of occult metastases in colon cancer. Dis Colon Rectum 46:221–231
Jung R, Petersen K, Kruger W et al (1999) Detection of micrometastasis by cytokeratin 20 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer 81:870–873
Champelovier P, Mongelard F, Seigneurin D (1999) CK20 gene expression: technical limits for the detection of circulating tumor cells. Anticancer Res 19:2073–2078
Vlems FA, Diepstra JH, Cornelissen IM et al (2002) Limitations of cytokeratin 20 RT-PCR to detect disseminated tumour cells in blood and bone marrow of patients with colorectal cancer: expression in controls and downregulation in tumour tissue. Mol Pathol 55:156–163
Soong R, Beyser K, Basten O et al (2001) Quantitative reverse transcription-polymerase chain reaction detection of cytokeratin 20 in noncolorectal lymph nodes. Clin Cancer Res 7:3423–3429
Jeffers MD, O'Dowd GM, Mulcahy H et al (1994) The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol 172:183–187
Greenson JK, Isenhart CE, Rice R et al (1994) Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer 73:563–569
Lassmann S, Bauer M, Soong R et al (2002) Quantification of CK20 gene and protein expression in colorectal cancer by RT-PCR and immunohistochemistry reveals inter- and intratumour heterogeneity. J Pathol 198:198–206
Rosenberg R, Hoos A, Mueller J et al (2000) Impact of cytokeratin-20 and carcinoembryonic antigen mRNA detection by RT-PCR in regional lymph nodes of patients with colorectal cancer. Br J Cancer 83:1323–1329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lassmann, S., Bauer, M., Rosenberg, R. et al. Identification of occult tumor cells in node negative lymph nodes of colorectal cancer patients by cytokeratin 20 gene and protein expression. Int J Colorectal Dis 19, 87–94 (2004). https://doi.org/10.1007/s00384-003-0530-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-003-0530-z