Abstract
Aim of the study
Human breast milk reduces the risk and severity of necrotizing enterocolitis (NEC). Exosomes are extracellular vesicles (EVs) found in high concentrations in milk, and they mediate intercellular communication and immune responses. The aim of this study is to compare the protective effects of exosomes that are derived from different time periods of breast milk production against intestinal injury using an ex vivo intestinal organoid model.
Methods
Colostrum, transitional and mature breast milk samples from healthy lactating mothers were collected. Exosomes were isolated using serial ultracentrifugation and filtration. Exosomes’ presence was confirmed using transmission electron microscopy (TEM) and western blot. To form the intestinal organoids, terminal ileum was harvested from neonatal mice pups at postnatal day 9, crypts were isolated and organoids were cultured in matrigel. Organoids were either cultured with exposure to lipopolysaccharide (LPS), or in treatment groups where both LPS and exosomes were added in the culturing medium. Inflammatory markers and organoids viability were evaluated.
Main results
Human milk-derived exosomes were successfully isolated and characterized. LPS administration reduced the size of intestinal organoids, induced inflammation through increasing TNFα and TLR4 expression, and stimulated intestinal regeneration. Colostrum, transitional and mature human milk-derived exosome treatment all prevented inflammatory injury, while exosomes derived from colostrum were most effective at reducing inflammatory cytokine.
Conclusions
Human breast milk-derived exosomes were able to protect intestine organoids against epithelial injury induced by LPS. Colostrum exosomes offer the best protective effect among the breast-milk derived exosomes. Human milk exosomes can be protective against the development of intestinal injury such as that seen in NEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is an inflammatory intestinal disease mainly found in 5–10% of premature and low weight birth infants [1]. Although considerable advancements in neonatal medicine were made, the mortality rate of necrotizing enterocolitis remains as high as 50%. With continuous studies on the contributing factors to NEC over the past several decades, pathogenesis of the disease remains poorly understood [2].
The advantages of human breast milk on infants born prematurely include reduction of occurrence of NEC [3]. Human milk is a dynamic body fluid which the bioactive compound composition changes through different lactation episodes. Although it is believed that these components are responsible for the beneficial effects of human breast milk in reducing the risk and severity of NEC, the mechanism of such protection has not yet been elucidated [4, 5].
Exosomes are extracellular vesicles (EVs) (30–150 nm) found in high concentrations in milk [6]. As a major cell–cell communication messenger, exosomes contain proteins, lipids and genetic materials which can be transported among cells to mediate intercellular communication and immune responses [7, 8]. Furthermore, exosomes can be isolated from human breast milk, and can be absorbed into intestinal epithelial cells to exert functions in vitro [9].
Recent developments in intestinal epithelial cell culturing have enabled a novel, more in vivo-like organotypic ex vivo model. These organoids resemble original tissue in structure and function [10, 11]. Through mimicking the natural microenvironment of the gut using the ex vivo model, the development of intestinal organoids may help to reduce animal experimentations and the rising failure rates in clinical trials [12]. This new approach has already been applied to drug metabolism and pathological studies, including NEC research. LPS administration to intestinal organoids decreased proliferation, while human milk was shown to exert a protective effect through downregulation of TLR4 (Toll-like receptor 4) [13, 14]. However, the effect of exosomes derived from human milk of different lactation episodes on intestinal organoid inflammation is unknown.
In this study, we sought to simulate intestinal injury using organoid model under conditions that mimic those in the infant gut environment, and to investigate the effect of the interaction of EVs derived from human breast milk with these three-dimensional (3D) injured intestinal organoids. In addition, we also aimed to compare the effects of breast milk-derived exosomes collected from different lactation periods at preventing intestinal organoid injury. We hypothesize that human breast milk exosomes exert protective effects on injured intestinal organoids through reducing inflammatory responses. Furthermore, we hypothesize that the protective effects of exosomes from different periods breast milk on intestinal injury is different.
Materials and methods
Human breast milk collection
Nine lactating mothers who had delivered preterm infants were enrolled between January 2019 and June 2019 at the Children’s Hospital, Fudan University. We collected colostrum (days 1–5 postpartum), transitional milk (days 6–14 postpartum) and mature milk (beyond day 15 postpartum) from these mothers (Table 1). All mothers were producing an excess of milk. Additionally, all mothers were over 18 years of age and healthy without any autoimmune conditions.
Exosomes isolation
Colostrum (n = 3), transitional (n = 3) and mature (n = 3) human breast milk was used. Exosomes were isolated from milk by serial centrifugations. All centrifugations were performed at 4 °C. Briefly, milk was centrifuged at 2000×g for 10 min to aspirate fat layer. Supernatant was transferred to a new tube, and centrifugation was performed again at 12,000×g for 40 min to eliminate cellular debris and somatic cells. Then, the new supernatant was filtered by 0.22 μm pore PES membrane through vacuum filtration. The exosomes were obtained from the filtered supernatant by ultracentrifugation at 100,000×g for 2 h at 4 °C using a Beckman Coulter L-90 K ultracentrifuge. After removing the exosome-free supernatant, the pellet was re-suspended in 500 μL phosphate buffered saline (PBS) in 1.5 mL Eppendorf tube, and then stored at − 80 °C until use for experiment.
Exosomes confirmation
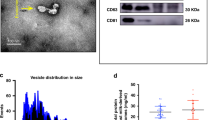
Exosomes were observed and micrograph images were taken by transmission electron microscope (TEM). Place the samples on formvar coated copper plate for 2 min, rinse with ultra-pure water, and dye 1% uranyl acetate with negative staining. Then the samples were observed by JEOL 1010 transmission electron microscope operated at 80-kV, and the images were captured with an Olympus soft-imaging Veleta digital camera.
Exosomes quantification
Protein concentration was quantified using a Bicinchoninic acid (BCA) reagent kit (Sigma Aldrich, Castle Hill, New South Wales, Australia). All 9 exosome samples collected from human breast milk were quantified against a bovine serum albumin (BSA) standard (0–2000 μg/mL) (Sigma Aldrich, Castle Hill, New South Wales, Australia) to determine protein concentration.
Intestinal organoids
An intestinal organoid model was established by isolating the crypts of terminal ileum harvested from neonatal C57BL/6 mice pups at postnatal day 9. Crypts were processed by multiple cycles of centrifugations and flushing, before suspension in 3D matrigel domes together with mouse organoid media (Stem Cell, IntestiCult Organoid Growth Medium). Lipopolysaccharide (LPS, Sigma, L2630) was added to the media to induce organoid epithelial injury, while in the treatment groups, 0.1 μg/μL of exosomes from either colostrum, transitional or mature human breast milk were also added together with the presence of LPS. These organoids were allowed to grow in different conditions for 48 h. Photomicrographs of organoids were taken by using a microscope IX71 with a DP72 camera (Olympus). The parameters (size and number of organoids) of each image were generated by the software Image J (1.52a version). Each value was presented as a mean of the analysis of at least three wells.
Gene expression
RNA was extracted from intestinal organoids using TRIzol (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions. RT-qPCR was performed to measure expression levels of inflammatory markers TNF-α, TLR4 and stem cell marker Lgr5 (Leucine-rich repeat-containing G-protein coupled receptor 5) to compare between control organoids, organoids exposed with LPS, and organoids treated with LPS plus exosomes. Results were generated from three independent experiments, each performed in technical triplicate. Expression levels were calculated by the ∆∆Ct method and normalized to the reference housekeeping gene GAPDH.
Immunofluorescence
Immunofluorescence staining for Ki67 was performed to assess proliferation capacity. For cultured organoid immunofluorescence staining, organoids were seeded on cover glass, fixed with 4% paraformaldehyde for 20 min at room temperature, and incubated with 0.5% Triton X 100 for another 20 min at room temperature. After blocking of non-specific binding, samples were incubated with primary antibodies Ki67 overnight at 4 °C, and with fluorescent secondary antibodies for 1 h at room temperature in dark condition. DAPI was added for visualization of cell nuclei. Immunofluorescent images of stained organoids were captured with a laser scanning confocal microscope
Statistical analyses
All data are presented as mean ± SD. *p < 0.05 and compared using one-way ANOVA with Bonferroni post-test.
Results
Exosomes were successfully isolated, confirmed and quantified
According to our primary confirmation through TEM images, exosome isolation was proven to be performed successfully. Bicinchoninic acid (BCA) quantified 5.4 ± 0.1 μg/μL for colostrum milk-derived exosomes, 4.1 ± 0.5 μg/μL for transitional milk-derived exosomes, and 3.8 ± 0.7 μg/μL for mature milk-derived exosomes. Purified breast milk exosomes were used in the following experiments.
Prevention of intestinal injury by administration of human milk-derived exosomes in ex vivo intestinal organoids
Gene expression levels of inflammatory markers TNF-α and TLR4 were significantly higher with LPS administration for 48 h (Fig. 1a, b). Milk exosomes effectively prevented the upregulation of these two genes induced by LPS. Furthermore, there were significant differences between colostrum, transitional and mature milk exosomes treatment at reducing inflammation. Among the three groups, exosomes derived from colostrum milk were most effective.
Exosomes prevented intestinal injury ex vivo. Relative expression of inflammatory markers TNF-α (a) and TLR4 (b) in intestinal organoids treated with LPS alone, LPS with 0.1 μg/μL of exosomes from colostrum, transitional or mature human breast milk, for 48 h. All human breast milk exosome administration prevented upregulation of both TNF-α and TLR4 induced by LPS, where exosomes from colostrum administration significantly decreased expression of TNF-α compared with transitional and mature milk. Experiments were independently repeated three times. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, using one-way ANOVA with post hoc tests
Exosomes prevented upregulation of intestinal regeneration induced by LPS
To analyze regeneration ability, organoids number showed no difference when comparing between control to LPS group to exosome treatment group (Fig. 2a, b). On the other hand, size of proliferating crypts is associated with the regeneration ability of the crypt cells [15]. In our study, we showed that LPS administration reduced the size of organoids, which means that such injury had a negative impact on intestinal regeneration (Fig. 2a, c). However, when exosomes were administered together with LPS, the size reduction was avoided (Fig. 2a, c).
Exosomes treatment prevented the change in morphology of organoids induced by LPS. Photomicrographs of organoids (a). There was no difference in response to LPS and exosomes in organoids number (b). LPS administration significantly decreased the size of organoids, and was prevented with exosome administration (c). Each value was presented as a mean of the tests of at least three wells. Data are presented as mean ± SD. *p < 0.05, using one-way ANOVA with post hoc tests
The expression of Lgr5, a marker of intestinal stem cell [15], revealed that LPS stimulated intestinal regeneration, possibly as a repair mechanism in response to LPS induced injury (Fig. 3c). However, when LPS was administrated with the presence of exosomes, the expression of Lgr5 remained low, similar to the level found in control group. Whereas there were no differences observed between exosomes derived from colostrum, transitional, and mature milk.
Exosomes hampered the upregulation of regeneration in injured intestinal organoids. Representative micrographs for Ki67 staining and corresponding quantification of fluorescence intensity in control, LPS, and exosome plus LPS treated organoids (a, b). Relative gene expression of intestinal stem cell marker Lgr5 in each experimental group (c). All human breast milk exosome administration prevented the upregulation of Ki67 and Lgr5 induced by LPS, whereas there were no differences between exosomes derived from colostrum, transitional and, mature milk. Experiments were independently repeated three times. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, using one-way ANOVA with post hoc tests
The results of immunofluorescence staining indicated that the proliferation marker Ki67 protein expression was increased in the LPS-induced injured organoids (Fig. 3a, b). We suspect that this again is a repair mechanism which the intestinal cells were trying to increase proliferation to replace the injured cells. And when LPS is administered with the presence of exosomes, Ki67 expression was shown to be lower than the injured group. However, there was no differences between exosomes derived from colostrum, transitional and, mature milk in suppressing the upregulation of Ki67 by LPS. Thus, human breast milk derived-exosomes treatments significantly hampered the injury induced by LPS administration.
Discussion
Studies have reported that exosomes can be isolated from human milk efficiently and can be absorbed by intestinal epithelial cells to exert functions [9]. In this study, we extended our investigations by mimicking the natural microenvironment of the gut through the development of the ex vivo organoid model, as well as to evaluate the effects of the interaction of human breast milk derived-exosomes with these organoids. On the other hand, bioactive compounds composition, including exosome concentration, of human breast milk varies during different lactation periods. Early milk, known as colostrum, contains a higher concentration of exosomes than mature milk. Interestingly, the onset of NEC occurs at 2–3 weeks of age, which might be when the mothers stop producing colostrum. Furthermore, it has been showed that administration of exosomes derived from milk collected from mothers that had delivered preterm infants significantly enhance proliferation and migration of intestinal epithelial cells compared to those of full term birth [16]. Thus, in this study, we isolated milk derived-exosomes from different lactation periods collected from mothers who delivered preterm infants.
It has been well established that inflammation in the intestine helps neonates defend against the invasion of pathogens under normal circumstances. However, the intestinal and immune system of NEC neonates are immature, which results in harmful excessive inflammation. In the present study, we were able to prove that human milk-derived exosomes treatment was able to hamper pro-inflammatory responses caused by LPS-induced injury, especially for exosomes derived from colostrum milk. Previous studies reported that the main functional component of milk exosomes is through the microRNAs found within these vesicles, and that the levels of different microRNAs varies among exosomes derived from different periods of milk [17, 18]. Thus, we suspect that the different effects between colostrum, transitional and mature milk on intestinal injury is due to their variation of microRNAs contained in exosomes. Besides, exosomes treatment significantly influenced TLR4 gene expression in the organoids. It has been reported that human breast milk was able to protect against NEC by inhibition of TLR4 through the epidermal growth factor receptor (EGFR) signaling pathway [19]. Further assessment of the effects of exosomes on the EGFR pathway can lead to the study of the mechanism of protection seen in our current study.
The intestinal regeneration capacity is crucial for repairing epithelium viability and integrity when intestinal injury was induced. Hock et al. reported that rat milk derived-exosomes promoted intestinal cell viability, enhanced proliferation, and stimulated intestinal stem cell activity [9]. Conversely, our study showed that exosomes administration together with LPS prevented the upregulation of proliferation and stem cell activity induced by LPS alone. We speculate that these differences can be explained by the varying injury severity. Previous study showed the capacity of regeneration is up-regulated in intestinal cells with minor injury, and is down-regulated with irreversible injury [20]. When a one stress factor alone, or when stress is given at low concentration, intestinal autonomous repair can be induced by upregulating regeneration mechanism. In our study, the protection by exosomes on intestinal organoids was able to prevent the activation of such autonomous repair. On the other hand, when high level of damage is induced, which might even disrupt or inhibit intestinal self-repair, we suspect that the effects by exosome as reported by Hock et al. will still be effective in rescuing the damaged epithelium, rather than protection as seen in our study. Thus, an organoid model with administration of more than one stress factors should be developed to aid in future studies focusing on using exosomes as a rescue treatment for NEC.
Exosomes isolated from human breast milk, especially colostrum, can be protective against the development of intestinal injury such as that seen in NEC. The onset of NEC occurs at 2–3 weeks of age when maternal colostrum is no longer produced. Given that this is the population most affected by NEC, prolonged administration of exosomes rich colostrum from milk bank present a possible preventive strategy against development of NEC in preterm infants.
Conclusion
This study contributes toward our understanding of the importance of exosomes as a major functional component in human breast milk. The observations provide us with insights on possible mechanisms underlying the role of breast milk in preventing severe intestinal diseases such as necrotizing enterocolitis. These results showed supporting evidences of using breast milk-derived exosomes as a promising therapy or nutrition strategy for such challenging disease.
References
Neu J, Walker WA (2011) Necrotizing enterocolitis. N Engl J Med 364(3):255–264
Zani A, Pierro A (2015) Necrotizing enterocolitis: controversies and challenges. F1000Res 4(F1000 Faculty Rev):1373
Section on B (2012) Breastfeeding and the use of human milk. Pediatrics 129(3):e827–e841
Lucas A, Cole TJ (1990) Breast milk and neonatal necrotising enterocolitis. Lancet 336(8730):1519–1523
Herrmann K, Carroll K (2014) An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med 9(4):184–190
Admyre C et al (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179(3):1969–1978
Thery C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15
Kalra H, Drummen GP, Mathivanan S (2016) Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17(2):170
Hock A et al (2017) Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg 52(5):755–759
Sato T et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265
Sato T et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5):1762–1772
Schweinlin M et al (2016) Development of an advanced primary human in vitro model of the small intestine. Tissue Eng Part C Methods 22(9):873–883
Kahn S et al (2018) Exosomal MicroRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res 62(11):e1701050
Lanik WE et al (2018) Breast milk enhances growth of enteroids: an ex vivo model of cell proliferation. J Vis Exp. https://doi.org/10.3791/56921
Barker N et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003–1007
Wang X et al (2019) Identification and peptidomic profiling of exosomes in preterm human milk: insights into necrotizing enterocolitis prevention. Mol Nutr Food Res 63(13):1801247. https://doi.org/10.1002/mnfr.201801247
Melnik BC et al (2016) Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab (Lond) 13:42
Liao Y et al (2017) Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res 61(11):1700082. https://doi.org/10.1002/mnfr.201700082
Good M et al (2015) Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 8(5):1166–1179
Lee C et al (2018) Influence of stress factors on intestinal epithelial injury and regeneration. Pediatr Surg Int 34(2):155–160
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81871849), the Science Natural Science Foundation of Shanghai (18ZR1405200), and the Critical Disease Joint Project of Xiamen city municipality (2501Z20179052).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, R., Zhang, R., Qian, T. et al. A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury. Pediatr Surg Int 35, 1363–1368 (2019). https://doi.org/10.1007/s00383-019-04562-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-019-04562-6