Abstract
Purpose
Ductal plate malformation (DPM) like arrays in the liver which resemble the characteristic persistent embryonal ductular structures have been shown to adversely affect the outcome of Kasai portoenterostomy (KPE) in biliary atresia (BA). We studied the impact of DPM on survival with native liver (SNL) in children with BA who underwent liver transplantation (LT) after KPE as well as those who underwent primary LT without KPE.
Methods
Records of children with BA who underwent LT in our institute were reviewed and divided into three groups—Group 1 had primary LT because of delayed diagnosis of BA and synthetic liver failure, Group 2 had LT for synthetic liver failure after a failed KPE, and Group 3 had LT despite clearing jaundice after KPE for other indications. The impact of DPM on SNL was analyzed using standard statistical means.
Results
In Group 1 (n = 26) and Group 2 (n = 26), the incidence of DPM was high and was associated with a significantly shorter SNL compared to children with no DPM. The incidence of DPM was significantly lower in Group 3 (n = 13).
Conclusion
DPM shortens SNL and influences the pathogenesis of disease progression in children with BA who had synthetic liver failure requiring transplantation either because of a failed KPE or due to a delay in diagnosis. Its incidence is low in children who cleared jaundice after KPE and needed transplantation for other indications at a later age. The presence of DPM signifies an adverse outcome for the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA) is a progressive fibrosing obstructive cholangiopathy of both the intra- and extra-hepatic biliary system. It has two forms, the perinatal or nonsyndromic form and the less common embryonal or syndromic form with associated extrahepatic developmental malformations known as ‘Biliary atresia splenic malformation’ (BASM) syndrome [1].
Ductal plate malformation (DPM) occurs due to failure of differentiation of the fetal biliary tract resulting in persistence of an excess of embryonic bile duct structures in the portal tracts [2]. The presence of circumferential biliary ductular structures within the connective tissue of portal tract in BA, which resemble primitive ductal structures or DPM, leads us to refer to these as “DPM-like arrays”.
Although the etiology of BA is multifactorial, the presence of DPM positive histology has been considered a marker of early intrauterine onset of disease and has also been associated with a worse outcome after KPE [3]. The aim of our study was to examine the impact of DPM on survival with native liver (SNL) in children with BA.
Materials and methods
Case records of children with BA who underwent LT in our institute over a 56 month period (August 2010–April 2015) were reviewed for demographic and clinical data. The presence of extrahepatic malformations (BASM) including polysplenia, preduodenal portal vein, cardiovascular defects, abdominal heterotaxy, intestinal malrotation, and abnormalities of the circulation of the liver was noted.
The patients were divided into three groups—Group 1 had a primary LT because of a delay in the diagnosis of BA and synthetic liver failure, Group 2 had LT for synthetic liver dysfunction after a failed KPE, and Group 3 had LT despite clearing jaundice after KPE. The indication for transplant in these children was recurrent cholangitis, hepatopulmonary syndrome, and portal hypertension.
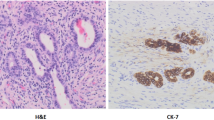
Histology of all the explants was reviewed by a single pathologist. All the available sections, which varied from 5 to 45 sections, were reviewed. These sections were stained with routine histopathological stains (hematoxylin and eosin), special stains (periodic acid–Schiff, periodic acid–Schiff with diastase, orcien, rhodanine, Perls iron, and Masson’s trichrome) and immunostaining with cytokeratin 19 (diluted at 1:50, CK 19, Biocare Medical, Concord, CA), a marker for ducts and ductules. Pressure cooking was used for antigen retrieval. All stains were performed on 4–5-μm-thick paraffin sections. The presence of morphological features such as DPM-like arrays, hilar and intraparenchymal bile cysts/bile lakes, and loss of bile ducts were recorded.
Both definite and possible DPM-like configurations were included in the study as described by Pacheco et al. [4]. They described definite DPM-like arrays as peripheral and concentric to portal vessels. Definite category included those arrays that were complete or almost completely circumferential. Possible DPM-like arrays were smaller compact ringlets eccentric to and/or unrelated to portal vessels making a tight arc around a small mesenchymal core with inconspicuous vessels (Figs. 1, 2). Bile cysts were noted as cystic structures containing bile casts with fibrous walls and ductal epithelial lining. Bile lakes were similar structures with lack of ductal epithelial lining (Fig. 3). The effect of DPM-like arrays on SNL in both groups was analyzed as was the association between DPM, bile cysts/lakes, and loss of bile ducts.
IBM SPSS Statistics v. 20 for Windows (IBM, Armonk, NY) was used for data entry and analyses. Continuous variables were analyzed using Mann–Whitney U test and Kruskal–Wallis test. Categorical variables were analyzed using Pearson’s Chi-square test. SNL was estimated using Kaplan–Meier method and compared using the Log rank test. A p value <0.05 was considered statistically significant for all tests of comparison.
This study was done after obtaining prior approval from the Institutional Review Board and clearance from the Ethics Committee.
Results
Of the 65 children (34 female, 52.3 %) with BA who underwent LT, 26 were in Group 1 (median age 11 months, range 5–49 months), 26 in Group 2 (median age 13 months, range 5–96 months), and 13 in Group 3 (median age 40 months, range 9–235 months). 59 of the children were of Asian origin and six were of African origin. 59 of the children underwent living donor LT and six underwent deceased donor LT. Only four children (6.2 %) had BASM. Overall median PELD Score was 15 (−10 to 28). The median PELD score in Group 1 was 17 (4–27), Group 2 was 17.5 (2–28), and Group 3 was −2 (−10 to 10) (Kruskal–Wallis test, p < 0.0001). Clinical and laboratory details of the children in the all groups are tabulated (Table 1).
Histopathological examination of explants in children who had LT for synthetic liver failure showed diffuse bridging fibrosis with complete micronodular cirrhotic transformation. The portal tracts and septa showed expansion with myxoid fibroplasia and florid ductular reaction (Fig. 4). In the explants of children who had cleared jaundice after KPE, large macroregenerative nodules around the hilum with preserved bile ducts were seen. Other histological findings identified in the sections were portal as well as septal polymorphonuclear and mononuclear inflammation, ductular cholestasis, cholangitis, hepatocyte rosetting, focal necrosis, lobular inflammation, syncytial multinucleate giant cells, hepatocyte ballooning degeneration, and Mallory-Denk bodies. Special stains for copper showed Grade 1–2 copper deposits. Kupffer cells showed stainable iron in few cases. Masson’s trichrome and Orcein stain showed bridging fibrosis to biliary cirrhotic transformation (Ishak score 4–6).
In the total group, DPM-like arrays was seen in 52.3 % (n = 34). All children with BASM (n = 4), and 30 (49.2 %) children without BASM had DPM. The incidence of DPM was higher in Group 1 (69.2 %, n = 18) and Group 2 (53.8 %, n = 14) compared to Group 3 (15.4 %, n = 2) (Chi-square test, p = 0.006).
Bile cysts/lakes were seen in 67.7 % (n = 44) of the explants. These cysts were located in the liver parenchyma as well as the hilum measuring 0.2–3 cm in maximum diameter and were filled with bile. Occasional multiple cysts were also identified. These cysts showed biliary sludge and variable inflammation with xanthogranulomatous inflammatory reaction which demonstrated sheets of foamy or ceroid-containing histiocytes associated with plasma cells, polymorphs, eosinophils, and occasional multinucleate giant cells. Foreign body type granulomas, characterized by aggregates of multinucleated giant cells, and foamy histiocytes, were seen around concretions of bile. This histological picture is quite different from cystic BA in which there is a cystic dilatation of the extrahepatic biliary tract at the liver hilum usually containing clear fluid as bile is unable to reach the cyst because of obliteration of hepatic ducts.
Loss of bile ducts was seen in 56 (86.2 %) explants. This finding was variable with some sections of the explant showing severe duct loss while other sections of the same explant showed ductular proliferation and DPM-like arrays.
In Group 1, DPM positivity was associated with a significantly shorter SNL (median age 9 months, range 5–20 months) as compared to children with no DPM (median age 20.5 months, range 13–49 months) (Mann–Whitney U test, p < 0.001). The median estimate of SNL in those with DPM was significantly lower at 9 months than those without DPM at 18 months (log rank test, p = 0.001) (Fig. 5).
Survival with native liver (SNL) in children with biliary atresia who had primary liver transplantation without Kasai portoenterostomy stratified by ductal plate malformation (DPM) positivity (n = 18) and negativity (n = 8). Those with DPM had significantly shorter SNLs compared to those without DPM
In Group 2 also, DPM positivity was associated with a significantly shorter SNL (median age 10.5 months, range 5–23 months) as compared to children with no DPM (median age 30.5 months, range 12–96 months) (Mann–Whitney U test, p < 0.0001). The median estimate of SNL in those with DPM was significantly lower at 10 months than those without DPM at 24 months (log rank test, p < 0.0001) (Fig. 6).
Survival with native liver (SNL) in children with biliary atresia who had liver transplantation for synthetic liver failure after Kasai portoenterostomy stratified by ductal plate malformation (DPM) positivity (n = 14) and negativity (n = 12). Those with DPM had significantly shorter SNLs compared to those without DPM
In Group 3, correlation between DPM and SNL was not possible due to the low incidence of DPM (15.4 %, n = 2) in this group.
30/34 children with DPM had bile cysts/lakes compared to only 14/31 children without DPM. This association was significant (Chi-square test, p < 0.0001). Loss of bile ducts was seen equally in those with DPM (28/34) as well as those without DPM (28/31) (Chi-square test, p = 0.353).
Discussion
Bipotential hepatic progenitor cells differentiate into periportal hepatocytes and cholangiocytes when they come into contact with the mesenchyme of the portal vein [2, 5]. These cells are arranged around the portal vein in a circular fashion and differentiate to form the primitive ductal structure finally maturing into tubular ducts [5]. Arrest in the differentiation process leads to persistence of an embryonic structure known as DPM [2]. In BA, the presence of circumferential biliary ductular structures within the connective tissue of portal tract, which resemble DPM, leads us to refer to these as “DPM-like arrays”.
Characteristic DPM is found in congenital cystic lesions of the intrahepatic bile ducts which include Von Meyenburg complexes, congenital hepatic fibrosis, polycystic liver disease, and Caroli’s disease [2]. Von Meyenburg complexes, also known as bile duct hamartomas or biliary microhamartomas, are benign malformations of the intrahepatic bile duct that occur due to the failure of embryonic involution of primitive ductal structures. Although they are part of the spectrum of adult polycystic diseases, these asymptomatic lesions are often identified at autopsy. The estimated incidence in some series is as high as 5.6 % in adults and 0.9 % in children [6].
The finding of DPM-like arrays in BA suggests an early developmental onset for the disease [3, 5]. In our series, DPM was seen in all children with BASM and 49 % without. Two other studies have also reported that DPM is not exclusive to the embryonic form of BA and does not identify this form of BA except that it may indicate an embryonic or perinatal disruption of ductular maturation [1, 4]. The etiopathogenesis of BA is multifactorial and no single etiology has been identified thus far but the final causal pathway involves cholestatic injury resulting in ductular destruction and proliferation. The presence of DPM in the liver of children with both perinatal and embryonic form of BA implies a role for this in the pathogenesis of both forms of the disease.
The overall incidence of DPM reported in most series is 20–50 % [3, 5, 7, 8], but these studies were done with single wedge biopsies of the liver taken at the time of KPE in which the large portal tracts are underrepresented leading to lower incidence. In our series, which looked at explant liver sections, it was high at 52 % because multiple sections with large portal tracts were available for study and, definitive and possible DPM-like arrays as defined by Pacheco were included. Since most children in our series had KPE elsewhere, the wedge biopsy of the liver at the time of KPE was not available to us to look for presence of DPM.
Our study showed that the presence of DPM had an impact on SNL in children with synthetic liver failure who underwent transplant after a prior KPE or underwent primary LT because of delayed diagnosis of BA. Children with DPM who had a prior KPE underwent transplant approximately 14 months prior to children without DPM. Similarly children with DPM who underwent primary LT for synthetic liver failure needed transplant approximately 9 months prior to children without DPM. The low incidence of DPM in children who had a successful KPE and needed transplant at a later age is also suggestive that DPM plays a role in disease progression of BA after KPE.
The impact of DPM on the outcome of KPE has been studied. Some authors have reported that DPM had a bad prognosis and in its presence, there is poor bile flow after KPE in infants with BA [7]. Arii et al. have shown that post KPE bilirubin was higher in the presence of DPM [8]. However, in their study, the presence of DPM did not influence the incidence of mid- or long-term complications including the necessity for liver transplantation.
Other factors such as early onset of jaundice and type of BA have been shown to have a significant impact on SNL in children who underwent KPE [5]. This information was not available in our series since all children had KPE in different centers. Also, due to this, variations in surgical technique of Kasai operation could have influenced the outcome of KPE, and hence SNL [9, 10]. There was no delay in transplantation as most patients in our series had grafts from living donors and adequate personal funding for LT. Hence SNL was not affected by time to transplant.
Multiple hypotheses have been proposed for the formation of bile cysts and lakes with some authors stating that cyst formation was secondary to the intrahepatic fibro-obliterative process leading to erosion and ulceration of the biliary epithelium resulting in bile leakage [11]. Few others proposed that the ongoing portal inflammatory process resulted in intrahepatic biliary radical obstruction, which caused cholangitis and bile cysts formation [12]. Some studies hypothesized that DPM lead to multiple cyst formation [13, 14]. In the presence of DPM, the bile duct injury is more. Although the incidence of DPM was very high in our series, it correlated well with the occurrence of bile cysts and lakes and is suggestive of a more exaggerated bile duct injury in the presence of DPM.
Conclusion
Children with DPM in the liver needed LT at an earlier age, irrespective of whether they had undergone a previous KPE. Thus, DPM influenced disease progression in BA and shortened SNL. Its incidence is low in children who cleared jaundice after KPE and needed transplantation for other indications at a later age. The presence of DPM signifies an adverse outcome for children with BA.
References
Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N (2006) The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 149:393–400. doi:10.1016/j.jpeds.2006.05.030
Desmet VJ (1998) Ludwig symposium on biliary disorders–part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc 73:80–89
Low Y, Vijayan V, Tan CE (2001) The prognostic value of ductal plate malformation and other histologic parameters in biliary atresia: an immunohistochemical study. J Pediatr 139:320–322. doi:10.1067/mpd.2001.117003
Pacheco MC, Campbell KM, Bove KE (2009) Ductal plate malformation-like arrays in early explants after a Kasai procedure are independent of splenic malformation complex (heterotaxy). Pediatr Dev Pathol 12:355–360. doi:10.2350/09-01-0598-OA.1
Vukovic J, Grizelj R, Bojanic K, Coric M, Luetic T, Batinica S, Kujundzic-Tiljak M, Schroeder DR, Sprung J (2012) Ductal plate malformation in patients with biliary atresia. Eur J Pediatr 171:1799–1804. doi:10.1007/s00431-012-1820-7
Redston MS, Wanless IR (1996) The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected]. Mod Pathol 9:233–237
Shimadera S, Iwai N, Deguchi E, Kimura O, Ono S, Fumino S, Higuchi K (2008) Significance of ductal plate malformation in the postoperative clinical course of biliary atresia. J Pediatr Surg 43:304–307. doi:10.1016/j.jpedsurg.2007.10.023
Arii R, Koga H, Arakawa A, Miyahara K, Lane GJ, Okazaki T, Urao M, Yamataka A (2011) How valuable is ductal plate malformation as a predictor of clinical course in postoperative biliary atresia patients? Pediatr Surg Int 27:275–277. doi:10.1007/s00383-010-2793-0
Davenport M, Ville De, de Goyet J, Stringer MD, Mieli-Vergani G, Kelly DA, McClean P, Spitz L (2004) Seamless management of biliary atresia in England and Wales (1999–2002). Lancet 363:1354–1357. doi:10.1016/S0140-6736(04)16045-5
Lampela H, Ritvanen A, Kosola S, Koivusalo A, Rintala R, Jalanko H, Pakarinen M (2012) National centralization of biliary atresia care to an assigned multidisciplinary team provides high-quality outcomes. Scand J Gastroenterol 47:99–107. doi:10.3109/00365521.2011.627446
Fain JS, Lewin KJ (1989) Intrahepatic biliary cysts in congenital biliary atresia. Arch Pathol Lab Med 113:1383–1386
Dillon P, Belchis D, Tracy T, Cilley R, Hafer L, Krummel T (1994) Increased expression of intercellular adhesion molecules in biliary atresia. Am J Pathol 145:263–267
Takahashi A, Tsuchida Y, Hatakeyama S, Suzuki N, Kuroiwa M, Ikeda H, Hirado J, Kitamura T, Matsuyama S (1997) A peculiar form of multiple cystic dilatation of the intrahepatic biliary system found in a patient with biliary atresia. J Pediatr Surg 32:1776–1779
Tsuchida Y, Honna T, Kawarasaki H (1994) Cystic dilatation of the intrahepatic biliary system in biliary atresia after hepatic portoenterostomy. J Pediatr Surg 29:630–634
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safwan, M., Ramachandran, P., Vij, M. et al. Impact of ductal plate malformation on survival with native liver in children with biliary atresia. Pediatr Surg Int 31, 837–843 (2015). https://doi.org/10.1007/s00383-015-3728-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3728-6