Abstract
The degree of viscero-abdominal disproportion often makes single-stage reduction difficult in large abdominal wall defects, without risking respiratory or hemodynamic compromise. As a consequence, clinicians have adopted a number of different methods to control these defects. Repair may be in the neonatal period, or later in life. Delayed repairs require epithelialization of the gastroschisis or omphalocele. Definitive repair may be in single or multiple stages. This paper describes four children in whom negative pressure wound therapy (NPWT) was used to facilitate closure of these complex defects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large congenital abdominal wall defects are a surgical challenge. No guidelines exist regarding the management of these complex cases, thus many different strategies are utilized [1]. Treatment strategies designed to close the abdominal wall defect after returning the viscera to the celomic cavity can be subdivided into neonatal and delayed. Neonatal repair may be primary or staged. Delayed repair requires epithelialization of the extracelomic contents in early life, followed by (usually) a staged repair sometime late in childhood. The degree of viscero-abdominal disproportion often makes it difficult to reduce the viscera in one stage at any age without respiratory or hemodynamic compromise [2, 3].
Negative wound pressure therapy (NWPT) is an accepted practice, with over 1,800 citations in the literature, and its safety in neonates and children has been demonstrated [4, 5]. While complete closure in congenital abdominal wall defects is the definitive goal, it is not always possible in the first instance. NWPT can decrease the complexity of closure procedures and act as staging posts along the path to closure [6]. Pre-operative reduction of extracelomic contents facilitates closure and allows monitoring of the effect on breathing and feeding. NWPT allows time for the child to slowly accommodate to the change in intra-abdominal and thoracic compartment pressures. This case series describes the use of NWPT to facilitate closure in four large, complex congenital abdominal wall defects.
Technique
A ring of hydrocolloid is applied to the abdominal wall surrounding the abdominal hernia; this allows for a more secure seal. Open weave gauze is then wrapped around the mass in a fashion similar to a head dressing; we use at least two rolls of Kerlix™ (Covidien, Mansfield MA, USA). An adhesive plastic dressing is applied to attain a seal. A port is then sited at the apex of the mass and the NPWT device switched on to check for a secure seal. We have not needed to change this dressing more frequently than once a week.
Case 1
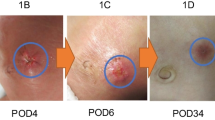
A female infant weighing 3.0 kg was born at 37 weeks gestation with a ruptured omphalocele. Initial management consisted of applying a silo (Bentec Medical Inc., Woodland CA, USA) and NPWT, followed by split-thickness skin graft. Due to the size of the omphalocele, she was unable to reach some of her developmental milestones, such as rolling and crawling. She was also unable to tolerate oral feeds and thus was fed via a nasogastric tube. At 8 months of age the patient was admitted for a trial of NPWT (Fig. 1a, b). Kerlix™ gauze was used as the interface, and the NPWT pump was applied at −40 mmHg continuous pressure. The patient was closely monitored for any increase in respiratory effort and difficulties feeding. Machine pressure was adjusted on day 1 to −80 mmHg and the dressings were reviewed on day 2, with more gauze applied for external pressure. After 9 days of treatment with NPWT, the abdominal hernia was found to be more pliable, softer and reduced in size (Fig. 1c). She was readmitted 1 month later for definitive surgical closure. On admission, NPWT was reapplied at −80 mmHg. The dressing was reviewed daily, with further external pressure applied by wrapping tape around the dressing. On day 7 of this admission, following external pressure being applied, the patient became irritable and tachypneic, with decreasing oxygen saturations. The tape was removed around the dressing and the patient stabilized. NPWT continued for seven more days with no further adverse incidents. After 14 days of NPWT, staged surgical closure was performed. The bowel was separated from the underside of the previous skin graft and the liver reduced into the abdominal cavity. The patient experienced decreased respiratory volumes upon fascial apposition and thus Surgisis™ mesh (Cook Biotech Inc, IN, USA) was applied rather than attempting complete closure. Mepitel™ (Mőlnlycke Health Care AB, Gőteburg, Sweden) and Acticoat™ (Smith and Nephew Medical, Hull, UK) were applied over the wound, with NPWT subsequently recommenced at −80 mmHg. The patient returned to the theater 9 days later, the mesh patch was removed and full fascial and skin closure obtained. NPWT was reapplied at −80 mmHg for support over the suture line for 1 week. The patient was discharged home with her family (Fig. 1d). Her oral intake had improved and she only required top-up nasogastric feeds. She was also able to tolerate tummy time. Her most recent follow-up visit was at 10 months post-closure. She is walking unaided. The repair is sound, though her transverse scar is unsightly (Fig. 1d). Further reconstruction may be required in the future.
a Prior to application of NPWT at 8 months of age, showing lateral and anterior views. b NPWT in situ, demonstrating molding and reduction of extracelomic contents. c Immediately after removal of NPWT after 9 days, showing laxity of skin with reduction of extracelomic contents. d 1 month post-fascial closure
Case 2
A female infant weighing 3.02 kg was born at 38 weeks gestation with a giant omphalocele. Initial treatment was NPWT and spontaneous epithelialization. At 15 months of age she was reviewed for repair of her abdominal hernia. NPWT was applied to reduce the hernia into the abdominal cavity prior to surgical reduction. Kerlix™ gauze was used as the interface, and the pump applied at −80 mmHg continuous pressure. The patient was managed as an outpatient with the dressing changed on day 2 and day 7; each time further external pressure was applied to reduce the umbilical hernia into the abdominal cavity. After 13 days of NPWT, the patient was taken to the theater for surgical reduction of the umbilical hernia. The redundant skin was excised, the liver reduced into the abdominal cavity and the fascial defect apposed. Reduction was well tolerated with no adverse events and complete closure was obtained. NPWT was reapplied over the suture line for support. The patient was discharged from hospital on day 2 post-surgery with a portable NPWT system and outpatient follow-up. The NPWT was removed on day 8 post-surgery, the suture line had healed and no further dressings were required. Follow-up has been maintained on a yearly basis with no further need for surgical involvement in the first two years after closure. Her next visit is due early in 2015.
Case 3
A male infant weighing 2.46 kg was born at 35 weeks gestation with gastroschisis. At birth the gastroschisis was partially reduced using a silo, and subsequent NPWT [7]. The abdominal contents were unable to be reduced over a 3 week period under NPWT, due to the degree of viscero-abdominal disproportion. The extracelomic organs were therefore covered with a split-thickness skin graft. At 9 months of age, NPWT was applied continuously at −80 mmHg over a large ventral hernia to reduce the hernia into the abdominal cavity. Kerlix™ gauze was again used as the interface. After 9 days of treatment, the patient was admitted for surgical reduction of the hernia. Redundant skin was excised and adhesions were divided. The bowel was returned to the abdominal cavity and full fascial and skin closure was obtained. The patient was discharged home on day 2. Dressings were removed 6 days post-operatively, and the suture line was healed with no further dressing requirements. The patient has been followed up annually and there have been no further surgical requirements 3 years post-closure.
Case 4
A male infant weighing 2.62 kg was born at 37 weeks gestation with omphalocele major. Primary closure was achieved at another hospital. Post-operatively the patient was noted to be anuric with increased ventilation pressures, and was therefore taken back to the operating theater where the closure was released and the extra-abdominal liver and gut placed in a pre-formed silo. Ongoing renal failure, at the time ascribed to abdominal compartment syndrome, led to the patient being transferred to our tertiary center for renal replacement therapy.
Over the course of his stay it became apparent that the patient had congenital renal disease, with both kidneys below the 5th centile for size. This required a change of strategy, as the child would necessarily be a candidate for renal transplant. NPWT was used at multiple points in this child’s care. Initially, his condition was too unstable to permit complex care of his abdominal defect. There were concerns that a pre-formed silo had an expected lifespan less than would be required in his case, so NPWT was used over the exposed liver and bowel. This enabled us to control the defect under a closed dressing, with cotside changes of dressing every 7–10 days.
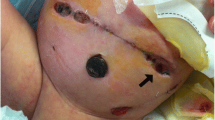
Given the difficulties experienced with central venous access for dialysis in this child, and the hemodynamic and coagulation instability caused by hemodialysis, peritoneal dialysis was attempted through the open abdomen. A dialysis catheter was sited surgically and NPWT applied over Mepitel™, Acticoat™ and Kerlix™ gauze (Fig. 2). A continuous infusion of dialysate was started, in the hope that it would remove fluid and electrolytes in its passage through the abdomen from the catheter tip up through the gauze and out via the NPWT suction tubing. This was partially successful, decreasing but not obviating the need for ongoing hemodialysis. Whether this was due to a failure of technique, or because the peritoneum was too inflamed to function as a dialysis membrane, is unknown.
Once the child’s condition had stabilized he was taken to the theater and a 4:1 meshed split skin graft was placed over the liver and bowel, following minimal debridement of granulation tissue with Versajet™ II (Smith & Nephew, Mt Waverley VIC, Australia). The previous NPWT had facilitated development of a layer of granulation tissue over the liver and bowel, facilitating graft take. NPWT was again used to hold the graft in place until incorporation [8].
NPWT was used in this patient prior to transplant. In this instance a negative pressure device was applied directly over the now epithelialized liver and bowel. As in the previous cases, extracelomic contents were gradually reduced into the abdominal cavity, acting as an autologous tissue expander. He was measured for a pressure garment at a comfortable point of reduction, and then NPWT was reapplied for 7 days until the garment was available. The patient was then placed in the garment. Our intention was that this would create the extra room required for a renal transplant, and that following this transplant we could dispense with the pressure garment and allow the gut and liver to ‘re-herniate’. This was successfully achieved and at 19 months old and 11.9 kg the child received an adult cadaveric transplant. The kidney is functioning well at the last review 10 months post-transplant. It is our intention to again utilize this method prior to formal reduction of his omphalocele and fascial closure, as in the previous cases presented. Our hope is that this approach will minimize the risk of increased intra-abdominal pressure compromising his transplant kidney.
Discussion
The primary aims of definitive fascial closure of large congenital abdominal wall defects are to allow the child to reach developmental milestones with an acceptable cosmetic result without respiratory or gut compromise [9]. In many cases it is not possible to achieve fascial closure in a single operation, either in the neonatal period or later in life [10]. This has led many pediatric surgical units to abandon primary closure in a single operation and instead adopt a staged approach to fascial reduction, either in the neonatal period or later in life [2].
Negative pressure wound therapy has rapidly found a place in wound management. It is not, however, a ‘one fix for all’ modality. There are many variables to consider when using NPWT—interface, filler, pressure settings, pressure cycling. The particular ‘recipe’ chosen from these variables is determined by the aims of treatment. This is best illustrated in the final patient, where NPWT was used with different aims at multiple points in his treatment. Initially, it was to cover and protect the wound while stabilizing the patient. Then we attempted to use NPWT as an adjunct to peritoneal dialysis. Following that, it was used to encourage a layer of granulation tissue over the liver, and subsequently to splint and stabilize the split skin graft. Finally it was used, as in the other patients, to commence reduction of extrafascial contents as an autologous abdominal tissue expander.
In the treatment of complex abdominal wall defects, there is no consensus regarding the best method of primary, staged or delayed reduction among pediatric surgeons. Perhaps the most potent example of this is the observation that 42 % of techniques published have subsequently been changed or abandoned by their original authors in this past half century [2].
One commonly employed method involves dissection of the fascial margin with placement of some form of mesh, which can then be gradually approximated over time until closure is possible. Just as this wound is under tension, there is also a tension between too little and too much reduction. A too rapid attempt at reduction can compromise respiratory, renal and enteral function, necessitating a relaxation of some of the gains achieved. Too slow a reduction risks the mesh eventually cutting out of the fascial margin, or infection entering the abdomen via this open wound. It also increases the number of occasions the child is subjected to general anesthesia.
Other forms of closure involve plastic surgical procedures designed to utilize the multiple muscle layers of the abdominal wall sliding or rotating one or more layers over the others to achieve closure. This is inevitably at the expense of other more lateral areas of the abdominal wall. It often involves extensive dissection of these areas and can leave the child with deficient abdominal wall musculature. It also requires specialist knowledge of muscle flaps if one is to minimize the risk of flap necrosis.
A third method involves the prior placement of tissue expanders [11]. While this may create extra room in the intra-abdominal domain, it does little to reduce the risk of Budd–Chiari syndrome from a too rapid rotation of a globular liver from outside to inside the abdomen [12]. Distinct from the above methods, it also requires a more complete exploration of the peritoneal cavity to allow placement of the expander.
Our technique obviates the need to implant an expander. Instead, in effect the extra-abdominal mass is an autologous tissue expander. A further advantage over implanted tissue expanders is that the extrafascial gut and liver are already reduced prior to surgery, by virtue of the pressure being applied by the pump. Reduction pressures can slowly be increased via pump pressures, and titrated to their effects on the patient. This technique means that reduction can be started prior to surgery, and outside of the operating theater. Gradual reduction of the liver prior to surgery may decrease the risk of Budd–Chiari syndrome.
Anecdotally, NPWT also appears to splint and stabilize the extracelomic contents, affording more comfort to the child and reducing the risk of vascular compromise of the extrafascial contents. NPWT can also be used to mold the extra-abdominal contents, depending on how gauze is applied. We can monitor the effects of reduction and the patient’s response to it. If the reduction is too rapid and the compartment pressures rise to a symptomatic level, it is a simple matter of decreasing the pump pressure on the NPWT. We also use NPWT application prior to surgery as a gauge of how likely we are to be successful in attempting fascial closure. If substantial reduction of liver and gut is possible without significant symptoms, then we can have more confidence in embarking upon staged surgery. Finally, NPWT can be reapplied following reduction and closure to splint the central abdomen. This final closure is inevitably under some tension; NPWT allows that tension to be redistributed over a greater surface area, rather than solely at the suture line. We postulate that it is largely this effect that is the reason for our anecdotal observation of increased wound comfort under NPWT.
Lessons learnt
-
1.
A good seal is important. A hydrocolloid ring provides a landing zone for the adhesive sheet of the NPWT.
-
2.
Cutting the adhesive sheet into isosceles triangles and applying sequentially around the circumference from the base of the ring to the apex give the best chance of attaining an immediate seal.
-
3.
Mold the NPWT complex as the pump pressure comes up. We believe a combination of lateral compression on the liver, and downwards pressure from the apex, allows the extracelomic mass to gradually dilate the hernia ring as it returns to the abdomen. This is analogous to a child’s head during labor and delivery. Sustained pressure is key.
-
4.
Once the extracelomic mass is reduced, the NPWT will to some extent draw the sides of the hernia ring together.
-
5.
Be patient; this may take some days to achieve reduction. Even partial reduction is beneficial toward closure.
-
6.
NPWT post-closure may splint the abdomen, decreasing analgesia requirements.
Conclusion
Large congenital abdominal wall defects are a surgical challenge. Due to marked viscero-abdominal disproportion, it is not always possible to achieve fascial closure in a single operation. The use of NWPT can facilitate closure in large, complex congenital abdominal wall defects by allowing extracelomic contents to act as an autologous tissue expander.
References
Levy S, Tsao K, Cox CS Jr, Phatak UR, Lally KP, Andrassy RJ (2013) Component separation for complex congenital abdominal wall defects: not just for adults anymore. J Pediatr Surg 48(12):2525–2529. doi:10.1016/j.jpedsurg.2013.05.067
van Eijck FC, Aronson DA, Hoogeveen YL, Wijnen RM (2011) Past and current surgical treatment of giant omphalocele: outcome of a questionnaire sent to authors. J Pediatr Surg 46(3):482–488. doi:10.1016/j.jpedsurg.2010.08.050
Foroutan H, Jenabali Jahromi B, Dastgheyb N, Najafi S (2013) A new method of repairing giant omphaloceles with bilateral mesh grafts lateral to the rectus abdominis muscles. Ann Colorectal Res 1(1):32–36. doi:10.5812/acr.11475
Choi WW, McBride CA, Kimble RM (2011) Negative pressure wound therapy in the management of neonates with complex gastroschisis. Pediatr Surg Int 27(8):907–911. doi:10.1007/s00383-011-2868-6
Morris MW Jr, Westmoreland T, Sawaya DE, Blewett CJ (2013) Staged closure with negative pressure wound therapy for gastroschisis with liver herniation: a case report. J Pediatr Surg 48(5):E13–15. doi:10.1016/j.jpedsurg.2013.03.005
Krug E, Berg L, Lee C, Hudson D, Birke-Sorensen H, Depoorter M, Dunn R, Jeffery S, Duteille F, Bruhin A, Caravaggi C, Chariker M, Dowsett C, Ferreira F, Martinez JM, Grudzien G, Ichioka S, Ingemansson R, Malmsjo M, Rome P, Vig S, Runkel N, Martin R, Smith J (2011) Evidence-based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury 42(Suppl 1):S1–12. doi:10.1016/S0020-1383(11)00041-6
Davies MW, Kimble RM, Woodgate PG (2002) Ward reduction without general anaesthesia versus reduction and repair under general anaesthesia for gastroschisis in newborn infants. Cochrane Database Syst Rev 3:CD003671. doi:10.1002/14651858.CD003671
Sakata S, Das GR, Leditschke JF, Kimble RM (2009) Extensive necrotising fasciitis in a 4-day-old neonate: a successful outcome from modern dressings, intensive care and early surgical intervention. Pediatr Surg Int 25(1):117–119. doi:10.1007/s00383-008-2289-3
Leppaniemi A, Tukiainen E (2013) Reconstruction of complex abdominal wall defects. Scand J Surg 102(1):14–19
Kilbride KE, Cooney DR, Custer MD (2006) Vacuum-assisted closure: a new method for treating patients with giant omphalocele. J Pediatr Surg 41(1):212–215. doi:10.1016/j.jpedsurg.2005.10.003
Adetayo OA, Aka AA, Ray AO (2012) The use of intra-abdominal tissue expansion for the management of giant omphaloceles: review of literature and a case report. Ann Plast Surg 69(1):104–108. doi:10.1097/SAP.0b013e31822128f5
Kaabachi O, Berg A, Laguenie G, Adamsbaum C, Bargy F, Helardot PG (1998) Budd–Chiari syndrome following repair of a giant omphalocele. Eur J Pediatr Surg 8(6):371–372. doi:10.1055/s-2008-1071236
Conflict of interest
The authors declare that they have no conflict of interest. No funding was sought or granted in the conduct of this study.
Ethical standard
This project has prior approval from the Children’s Health Services Queensland Human Research Ethics Committee and has therefore been conducted in concordance with the principles of the Declaration of Helsinki. Consent has been obtained from parents for inclusion of their children’s stories and images within this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McBride, C.A., Stockton, K., Storey, K. et al. Negative pressure wound therapy facilitates closure of large congenital abdominal wall defects. Pediatr Surg Int 30, 1163–1168 (2014). https://doi.org/10.1007/s00383-014-3545-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3545-3