Abstract
The issue of antenatal hydronephrosis has become a routine component for the care of a pregnant woman despite limited evidence of a clinical benefit. The genitourinary tract represents the most commonly detected organ system with identified abnormalities, with antenatal hydronephrosis (ANH), being the most notable and common finding. ANH represents a spectrum, with most cases being a trivial and inconsequential finding on maternal fetal ultrasound. However, there is a correlation with increased grades of ANH being associated with increased severity of urinary tract pathology. Most patients can be managed expectantly with appropriate evaluation commenced postnatally based on severity of ANH and proper parental counseling and education. The purpose of this review was to assess current literature and guidelines pertaining to ANH and incorporate our practical interpretations of their significance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the late 1970s, prenatal screening with ultrasound has become a routine component of care for pregnant women worldwide. Studies have found that approximately 1 % of ultrasounds detect fetal anomalies. Of these detected anomalies, genitourinary abnormalities are amongst the most common, accounting for 20 % of identified anomalies. Furthermore, antenatal hydronephrosis (ANH), defined as dilation of the fetal renal collecting system, affects between 1 and 5 % of pregnancies [1].
The differential diagnosis of antenatal hydronephrosis is quite broad ranging from ureteropelvic junction obstruction, vesicoureteral reflux, and posterior urethral valves. Left untreated these pathologies may result in postnatal morbidity including nephrolithiasis, urinary tract infection, renal scarring and ultimately, renal loss, and chronic kidney disease. Early diagnosis and further postnatal investigation of these anomalies have the potential to reduce morbidity [2]. Yet, the vast majority of ANH is transient in nature and resolves spontaneously without intervention or complication, and hence is a benign, yet worrisome peculiarity. Given this fact, it becomes important to realize that hydronephrosis represents a spectrum, and as clinicians, we are mandated to differentiate which cases of hydronephrosis present the greatest risk for developing postnatal pathology and require follow-up and potential intervention. Given the immense differences in parental angst, education, perception, and even availability of medical care, the proper counseling and education of the parents becomes paramount in the decisions to investigate and treat their new child with ANH.
Current practice regarding the evaluation and treatment of children with ANH remains in flux within the pediatric urology community and is far from uniform and often is based on dogma, training, and personal or institutional bias. Although algorithms have been devised to investigate the infant with ANH, none are perfect for each and every patient that is referred for evaluation.
This manuscript reviews the primary literature and consensus statements pertaining to ANH and sets forth our own recommendations regarding management of infants with this finding. The vast majority of this work is based on upper tract pathology relating to ureteropelvic junction obstruction as the subject is far too immense to similarly review all causes of ANH
Natural history of antenatal hydronephrosis
Prenatal detection of ANH
Prenatal ultrasound screening is most commonly performed at 18–20 weeks gestation, which also coincides with the point at which renal architecture becomes visibly distinct. The most commonly utilized parameter for determining the presence and severity of ANH on prenatal screening is the anterior–posterior diameter (APD) of the renal pelvis. There is as of yet no predetermined APD value which discriminates pathological from benign ANH [3]. Establishing such a threshold is difficult because of variation in APD associated with a number of factors including gestational age and maternal hydration status.
Multiple studies have examined the APD measured on prenatal ultrasound necessary to predict postnatal pathology. One such study by Ismaili et al. prospectively followed 213 infants and found that an APD cut off of 10 mm in the third trimester would have detected only 23 % of renal anomalies. Comparatively, an APD cut off of 7 mm in the same patient cohort detected 68 % of abnormalities suggesting that a lower APD cut off provides greater sensitivity in detecting pathology [4]. A similar study conducted retrospectively by Coplen et al. [5] evaluated a cohort of 257 neonates with prenatally detected hydronephrosis and found that an APD cut off of 15 mm detected renal pathology in approximately 80 % of fetuses with a sensitivity of 73 % and a specificity of 82 %.
Classification of antenatal hydronephrosis
The classification of ANH by severity of disease is an integral aspect of the clinical management of ANH as this helps estimates the risk of potential pathology and guide clinical decision-making. A number of grading systems have been utilized to classify ANH, but they are all complicated by subjectivity and inter-provider variability. In order to overcome this subjectivity, more objective parameters have been implemented, namely APD. Lee et al. performed meta-analysis of 17 studies and a total of 1,308 subjects with antenatal hydronephrosis and were able to stratify ANH based on the size of the APD on prenatal ultrasound. Their analysis also found a difference in APD threshold based on gestational age. For instance, mild disease was categorized by an APD ≤7 mm in the second trimester and ≤9 mm during the third trimester. Similarly, severe ANH was defined as an APD ≥10 in second trimester ultrasounds and ≥15 mm in the third trimester [6].
Only rarely does antenatal hydronephrosis of any degree progress with such rapidity to require fetal intervention to relieve urinary obstruction. Animal models have shown that urinary obstruction not only results in renal dysplasia and kidney failure, but due to decreased amniotic fluid, normal pulmonary development is impeded [7–10]. Currently, intervention, such as open fetal surgery, vesicocentesis or renal pelvis aspiration, is reserved for fetuses with solitary kidney and severe hydronephrosis and oligohydramnios or in fetuses with posterior urethral valves and oligohydramnios. Intervention is only recommended in the second and third trimesters and carries significant morbidity and mortality limiting its utility.
While APD measurement provides an objective means of predicting pathology, most in the field would agree that other features are also important in determining severity of this finding. Therefore, features such as calyceal dilation and parenchymal thinning should be considered in grading the severity of ANH. In establishing their own grading system the Society for Fetal Urology (SFU) took these factors into account (Fig. 1). The system grades hydronephrosis on a five-point scale with grade 0 representing normal renal ultrasound, grade 1 demonstrates the onset of hydronephrosis, and grade 4 hydronephrosis with dilation of the pelvis and major calyces in addition to thinning of the parenchyma [2].
The Society for Fetal Urology Grading System of Antenatal Hydronephrosis (ANH). This figure depicts the five grades of ANH ranging from grade 0 which is a normal kidney to grade 4 where there is the most severe pelvicalyceal dilation with a reduction in renal parenchyma. There is clear relationship with increased SFU grades of ANH to more severe pathology
Long-term outcomes
To best grasp the natural history of antenatal hydronephrosis and predict pathology for newly diagnosed cases, it is important to understand the long-term course of patients in prospective studies. These studies, however, are limited in their scope as most discontinue follow-up in the early years of childhood. Perhaps the best data about long-term outcomes in patients with antenatal hydronephrosis come from the anecdotal accounts of Dhillon [11] and the experience at the Great Ormond Street Hospital in London and as further described by Thomas [12].
In one cohort of 76 children with prenatally detected ANH all were found to have significant renal function as demonstrated by values >40 % and were observed for a minimum of 16 years. Of these 76 children, 52 % experienced significant or complete resolution of hydronephrosis without recurrence in the follow-up period. Another 11 % of the patients had stable hydronephrosis throughout the follow-up period without complication or intervention. Finally, 37 % eventually underwent pyeloplasty for increased dilation, decreased differential function or onset of symptoms such as infection [11, 12].
These results are similar to those from other observational studies with shorter follow-up time. Ulman et al. followed 104 neonates with severe unilateral ANH and found that 23 children eventually required surgery. Of the remaining children, 69 % experienced spontaneous resolution of hydronephrosis within an early follow-up period of <2.5 years. Another 31 % had persistent but improved hydronephrosis in this same follow-up period. Furthermore, of those children initially found to have decreased differential function of <40 %, 24 % had an improvement in function to a mean of 47 % by 18 months [13]. Ismaili et al. published retrospective data for a cohort of 234 neonates with antenatally detected hydronephrosis over a longer period of time, up to 13 years. In this cohort, 22 % required early pyeloplasty for reduced function. The remaining 182 children were managed conservatively with observation and 137 were found to have stable or improved renal function. Delayed pyeloplasty was performed in 45 of the 182 neonates for decline in differential function or UTI at a mean age of 18 months [4, 14]. Unlike the Dhillon data, the Ismaili group did not describe a mean or median length of follow-up with which to better interpret the data.
Observation versus early intervention
The long-term prospective and retrospective data demonstrate that the majority children with antenatally diagnosed hydronephrosis will have spontaneously resolving dilation or remain asymptomatic with persistent dilation. However, approximately 25–33 % of cases will worsen over the course of observation with decreased renal function or infection and require surgical intervention. With this data in mind, it then becomes imperative to design a course of management that prevents over-use of unnecessary surgery but remains sensitive enough to detect cases of declining function. Organizations such as the Society for Fetal Urology (SFU) and Canadian Urological Association (CUA) have put forth recommendations to guide practitioners, but the community remains divided on their interpretation of the available data to be reviewed below [2, 15].
Antibiotic prophylaxis
There has long been an argument that children with ANH should be placed on antibiotic prophylaxis to prevent UTI and potential renal damage from pyelonephritis. As of date, there have not been any prospective randomized trials evaluating the utility of prophylactic antibiotics in children with ANH.
There are multiple conflicting retrospective studies, some showing an increased risk of UTI and others not, and the topic remains controversial.
A retrospective cohort analysis by Walsh et al. [16] found that infants with hydronephrosis were 12 times more likely to be hospitalized for pyelonephritis in the first year of life compared with infants without hydronephrosis. Other groups have found similar results in their analysis and also demonstrated that the risk of infection increases with the grade of hydronephrosis. In their analysis of a cohort of 192 infants with ANH, Coelho et al. [17] found an associated incidence of infection with mild, moderate, and severe hydronephrosis was 11, 18, and 36 %, respectively, at the age of 36 months. Lee et al. found similar results indicated that the rate of infection approached 40 % in neonates with SFU grade 4 hydronephrosis, even when controlling for reflux [18]. In both these studies urinary tract infections were diagnosed by bagged urine specimens which may have over estimated the incidence of UTI.
There are an equal number of studies that demonstrate a low risk of urinary tract infections in children with ANH without vesicoureteral reflux. Estrada et al. [19] focused their analysis on 1,514 out of 2,076 with ANH that had grade 2 hydronephrosis who were screened for VUR with voiding cystourethrogram. Of the 828 patients who did not have reflux, only 11, or 1.3 % ultimately developed UTI. Focusing on 92 patients with grade 3 or 4 hydronephrosis without reflux, Roth et al. found a history of UTI in only 4.3 % of children. The data from this study show a slightly higher rate of UTI, 8.3 %, in children with hydroureter, but still lower than the above studies [20].
Analyzing the available data, including those presented above, the official of recommendations of the Canadian Urological Association (CUA) recognize the ambiguity surrounding the issue and only confer a grade D recommendation for the use of antibiotic prophylaxis for cases of hydronephrosis without reflux [15]. Conversely, the SFU has drafted recommendations for the use of prophylactic antibiotics in all cases of hydronephrosis except for the most mild. Similarly, the SFU recommends antibiotic use for children with additional risk factors for UTI such as hydroureter and reflux [2]. Each set of guidelines recognizes that antibiotic use is not without risk, including various side effects, such as allergic reactions, development of bacterial resistance, and increased risk of yeast infections. Given the uncertainty of the available data, the decision to begin antibiotic prophylaxis is one that should be made with careful consideration of the potential risks and benefits. Many within the field, including the authors, would argue that prophylaxis should be deferred in exchange for a lower threshold to initiate treatment of potential UTIs, especially in children with low-grade hydronephrosis.
Circumcision
Perhaps even more ambiguous and controversial than the recommendations regarding the use of antibiotics in children with antenatal hydronephrosis, is the role of circumcision in the prevention of infection in these patients. The procedure is one with deep religious and cultural roots which vary worldwide and must be factored into decisions regarding patient management. Moreover, its position in medical practice has waxed and waned over the course of the past several decades. Most recently, the American Academy of Pediatrics increased their support of the procedure given the evidence of preventing urinary tract infection, HIV transmission, and penile cancer, but does not recommend routine circumcision for all newborns [21].
Several studies have shown that circumcision can prevent urinary tract infection in with reflux and posterior urethral valves. For instance, Mukherjee et al. performed retrospective cross-sectional analysis of 78 uncircumcised patients with PUV. Of these boys, 27 were circumcised and experienced an 83 % reduction in the incidence of UTI when compared with uncircumcised boys. More importantly, the study also found that the NNT for prevention of UTI with circumcision in boys with PUV is close to 1, compared with 111 for boys with GU abnormalities [22]. Herndon et al. found similar results in their multicenter study of children with vesicoureteral reflux. Their study analyzed data from a total of 56 boys, 37 of whom where circumcised and the remaining 19 were uncircumcised. Of these boys, those who were uncircumcised had a higher incidence of infection, odds ratio of 4.0, with 35 % of developing UTI despite being on prophylactic antibiotics, compared with only 19 % of circumcised boys [23]. The data for children with hydronephrosis without hydroureter or PUV is less prolific. In their analysis of children with grades 3 and 4 hydronephrosis, Roth et al. demonstrated that none of the circumcised children developed UTI when compared with 8.3 % of uncircumcised boys who did develop infection [20].
All of the data suggest that boys with any grade of hydronephrosis would benefit from circumcision. However, the studies are all retrospective studies and to date, there have been no prospective randomized trials to verify the utility of circumcision. Such studies are likely unlikely in the future given the delicate and personal nature of the procedure in families of these children. It is precisely because of this that the decision to pursue circumcision must be individualized to each child and family.
Renal ultrasound
The review of the literature demonstrates that renal ultrasound is an important means of evaluating infants with ANH both in both initial and follow-up phases of care. As a screening tool, ultrasound allows for the differentiation of low- and high-risk disease based on SFU grading criteria of 0–2 and 3–4, respectively. Those patients which are determined to be low-risk continue to require interval follow-up imaging and given the absence of radiation, ease of use and non-invasiveness, this is most effectively done by ultrasound. The data demonstrate that monitoring SFU criteria and measuring APD can help predict which patients will require surgical intervention and guide further treatment.
The vast majority of ANH is detected during the second trimester of pregnancy as anatomical screening via ultrasound typically occurs at 20 weeks gestation. Abnormal findings on this initial scan are then generally followed with repeat prenatal ultrasound during the third trimester and almost always with renal ultrasound during the postnatal period. The renal ultrasound has become the most common imaging modality in the evaluation and follow-up of antenatally diagnosed hydronephrosis as indicated by the recommendation of the SFU for use in all cases of ANH and a grade A recommendation from the CUA [15].
Renal ultrasound confers a number of benefits as a tool for postnatal evaluation of ANH. Ultrasound is absent of radiation, relatively easy to use, and readily accessible in most locations. However, like most medical technologies, it is not without flaws. There is a high degree of inter-operator variability in skill and interpretation which can decrease predictive value [24]. Additionally, hydration status of the infant can also affect the ability of ultrasound to predict pathology. At birth, infants are relatively dehydrated and therefore an ultrasound performed immediately after birth can underestimate the degree of hydronephrosis [24]. Therefore, multiple organizations including the SFU and CUA recommend postponing the initial postnatal renal ultrasound until at least 1 week after birth unless necessitated by symptoms such as febrile infection or rising creatinine [2, 15]. These same guidelines propose increased vigilance in children with additional risk factors for renal damage including those with severe bilateral hydronephrosis and any grade of hydronephrosis in a solitary kidney by recommending ultrasound be performed prior to discharge from the hospital at birth.
Ultrasound is a screening modality that may determine the need for further studies to evaluate the need for surgical intervention. Moreover, serial ultrasounds can determine progression of dilation and renal dysfunction. The CUA recommends using ultrasound in conjunction with SFU grading criteria to classify patients into observational groups and cases requiring additional evaluation. SFU grades 0–2 can be observed closely with annual imaging to detect worsening of hydronephrosis, but more severe disease, grades 3–4, often necessitate a more extensive work-up [15]. Similarly, Dhillon and associates at the Great Ormond Street Hospital found that measuring APD can help predict the need for surgical intervention, prospectively following 139 renal units with differential function >40 % over a period of 6–13 years with conservative management. For kidneys with an APD between 30 and 40 mm, 21 out of 25 eventually required surgery to correct obstruction. Additionally, they followed 36 kidneys with an APD >40 mm and found that all of them eventually required surgical correction [11].
Voiding cystourethrogram
Voiding cystourethrogram has traditionally been a standard component in the evaluation of infants with ANH to detect vesicoureteral reflux and lower tract pathology such as posterior urethral valves, ureteroceles or bladder diverticula. However, insight into the natural history of ANH from long-term studies showing that the vast majority of cases resolve or remain asymptomatic has instigated a new debate on the utilization of VCUG [14, 19, 23, 25, 26]. Both the Society for Fetal Urology and Canadian Urological association have recommended that all cases of ANH found on renal ultrasound to have SFU grade 4 dilation undergo VCUG to rule out reflux and other potential pathology. Similarly, the organizations also recommend that VCUG be deferred for less severe cases of ANH, SFU grades 0–2, as the modality is more invasive than ultrasound and these children have not been shown to progress to significant pathology [2, 15]. The recommendations become equivocal, however, when dealing with ANH classified as SFU grade 3 as this group of kidneys has the most conflicting data regarding progression of pathology. Because of this ambiguity, it is recommended that a more individualized approach be taken with these patients and the decision to pursue VCUG be made on a case by case basis and with parental involvement in the decision making. Factors which would influence the decision to recommend VCUG include any findings which suggest lower urinary tract disease like posterior urethral valves. These findings include bilateral hydronephrosis, dilated ureter, duplex kidney, abnormal renal echogenicity, and abnormal appearance of the bladder.
Renal function scan
In evaluating patients with ANH, renal scintigraphy can offer helpful information to plan further treatment including an estimation of renal function and a diagnosis of obstructive pathology. However, as with VCUG, the use of renal scan in the indications for employing the technology remains controversial because of its invasive nature, cost, and radiation exposure. Furthermore, there are a number of different radiopharmaceuticals—including Tc-MAG 3, Tc-DTPA, and Tc-DMSA—used for renal scan that further complicate the decision process. Of these three isotopes, Tc-MAG3 has become the most frequently used and standardized agent as it provides improved definition of parenchymal and collecting systems to better estimate renal function and requires less radiation exposure [27]. Moreover, it can be performed without sedation or anesthesia in the very young patient. However, even in the “best of hands”, the dynamic scans are open to interpretation and are imperfect. Hydration, bladder distention, renal capability and function to respond to furosemide are all important parameters in assessing drainage. In fact, many practitioners look as much if not more, at the initial perfusion or function data, than the response curves to diuretic. As such, it becomes a “poor man’s” DMSA scan. Still, if there is prompt washout of tracer, then one feels more comfortable in following a surveillance protocol.
In patients with lower risk disease, SFU grades 0–2, renal scan assumes a role similar to that of VCUG and is only pursued when observation or increased symptoms demonstrated progression of hydronephrosis. For SFU grade 4 hydronephrosis and VCUG showing no reflux, a MAG 3 renal scan is recommended to determine renal function and drainage. If reflux is present and especially if high grade a DMSA scan may be beneficial as a large majority of these children have renal dysplasia.
For grade 3 hydronephrosis the management should be individualized and decision made with parental involvement. We would recommend serial ultrasound and reserving the MAG 3 scan for those without reflux and with persistent or increasing hydronephrosis. As the diuretic phase of the MAG 3 renal scan is not accurate in determining obstruction the absolute indication for surgical intervention recommended by the SFU is a decrease in differential renal function to <40 %. Other indications for surgery include increasing hydronephrosis on follow-up with delayed drainage on renal scan [27, 28].
Summary and recommendations
Antenatal hydronephrosis is the most commonly diagnosed anomaly on prenatal ultrasound. Meta-analysis has shown that these children have an increased risk of pathology postnatally when compared with children within the normal population. However, the degree of this risk, like the severity of hydronephrosis, varies largely between children. Historically, most children with antenatal hydronephrosis were managed surgically to prevent potential adverse outcomes such as infection and renal failure. Recently, however, this practice has become less common as data from long-term follow-up, such as that reviewed above, have begun to suggest that the majority of children will remain asymptomatic with stable or resolving hydronephrosis. Our review also evaluates the data available on the sensitivity and specificity of a number of imaging modalities in predicting pathological antenatal hydronephrosis. While every child must ultimately be managed in an individual manner, these data provide an ideal framework upon which to base management guidelines for infants with antenatal hydronephrosis.
Recommendations
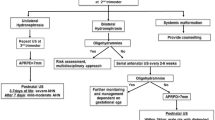
SFU grades 0–2
Long-term data indicate that infants with low-grade antenatal hydronephrosis have resolution of dilation or remain stable without pathological complication in the majority of cases. Consequently, initial surgical intervention is not indicated or recommended by either the SFU or CUA. Rather, a protocol of expectant management should be initiated to monitor for any progression of dilation or development of symptoms that may lead to future renal damage such as fever. As per the recommendations of both the SFU and CUA, all infants with antenatally diagnosed hydronephrosis should have a renal ultrasound shortly after birth, but no sooner than 2 weeks of life to avoid the initial postnatal diuretic phase.
SFU grade 3
We would recommend antibiotic prophylaxis until the studies are completed, particularly if the family chooses to pursue a VCUG. Ultrasound should be performed at 7–14 days after birth in an otherwise healthy infant. We would counsel the family on performing a VCUG to evaluate for reflux. If VCUG is negative for reflux we would recommend discontinuing antibiotic prophylaxis. If no VCUG is performed, again a frank discussion with the family is in order, and a decision made after providing the data available and respecting their sense of comfort. Circumcision also becomes a decision based on similar models.
We would recommend repeating the ultrasound at around 3 months of age and if the degree of hydronephrosis remains the same or worsens a Tc-MAG 3 diuretic renal scan should be performed.
SFU grade 4
These children should be placed on prophylactic antibiotics until the studies are completed. Particularly, if there is ureteral dilation, a VCUG should be encouraged. If the VCUG shows no reflux antibiotics may be discontinued, even realizing that the retrospective data suggests an increased risk of UTI in this group of children.
Children with grade 4 hydronephrosis have the most severe renal anomalies and, as shown in the above long-term follow-up data, the greatest risk for developing renal pathology. Moreover, these data also show that these children most often require surgical intervention to prevent said adverse events. As with all infants with ANH, it is our recommendation, in addition to that of the SFU and CUA, that renal ultrasound should be performed after 2 weeks of life to reassess renal dilation. Furthermore, because of the increased risk of pathological outcomes in these children, VCUG should be encouraged. If vesicoureteral reflux is found a DMSA scan may be offered in selected cases in order to evaluate differential function. If the VCUG is negative for reflux a diuretic renogram should be performed to elucidate etiology of hydronephrosis and plan for potential surgical management. Again, because of the conflicting data regarding antibiotic prophylaxis and circumcision in children with ANH, we recommend that they be reserved for symptomatic cases.
Hydroureter
In this group of patients, the risk of developing urinary tract infection is greater than in children with dilation limited to the kidney. Therefore, greater care must be taken to protect against renal damage. It is our recommendation, and that of the SFU, that these children undergo imaging within the first 7 days, including at least a renal ultrasound. Similarly, these patients should also be placed on antibiotic prophylaxis until imaging studies are complete because of their increased risk of infection.
Bilateral hydronephrosis
As with hydroureter, there is new evidence, which, while limited, suggests that children bilateral hydronephrosis may benefit from early evaluation and antibiotic prophylaxis. As described above, children with bilateral disease are at increased risk of infection compared with children with unilateral hydronephrosis. This risk also increases with the grade of hydronephrosis with bilateral severe hydronephrosis having higher incidence of infection than milder cases. Therefore, we recommend, along with the SFU and CUA, that these children be placed on antibiotic prophylaxis while awaiting studies to be completed. Bilateral hydronephrosis not only carries an increased risk of infection, it may also be a sign of more severe underlying pathology such as posterior urethral valves in boys. Therefore, it is recommended that these children be evaluated with ultrasound and potentially VCUG prior to being discharged from the hospital after birth.
References
Mallik M, Watson AR (2008) Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol 23:897
Nguyen HT, Herndon CD, Cooper C et al (2010) The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 6:212–231
Ismaili K, Avni FE, Piepsz A et al (2004) Current management of infants with fetal renal pelvis dilation: a survey by French-speaking pediatric nephrologists and urologists. Pediatr Nephrol 19:966
Ismaili K, Hall M, Donner C et al (2003) Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol 188:242
Coplen DE, Austin PF, Yan Y et al (2006) The magnitude of fetal renal pelvic dilatation can identify obstructive postnatal hydronephrosis, and direct postnatal evaluation and management. J Urol 176:724
Lee RS, Cendron M, Kinnamon DD et al (2006) Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics 118:586
Harrison MR, Golbus MS, Filly RA et al (1987) Fetal hydronephrosis: selection and surgical repair. J Pediatr Surg 22:556
Harrison MR, Golbus MS, Filly RA et al (1982) Fetal surgery for congenital hydronephrosis. N Engl J Med 306:591
Harrison MR, Golbus MS, Filly RA et al (1982) Management of the fetus with congenital hydronephrosis. J Pediatr Surg 17:728
Harrison MR, Nakayama DK, Noall R et al (1982) Correction of congenital hydronephrosis in utero II. Decompression reverses the effects of obstruction on the fetal lung and urinary tract. J Pediatr Surg 17:965
Dhillon HK (1998) Prenatally diagnosed hydronephrosis: the Great Ormond Street experience. Br J Urol 81(Suppl 2):39
Thomas DF (2010) Prenatal diagnosis: what do we know of long-term outcomes? J Pediatr Urol 6:204
Ulman I, Jayanthi VR, Koff SA (2000) The long-term followup of newborns with severe unilateral hydronephrosis initially treated nonoperatively. J Urol 164:1101
Ismaili K, Hall M, Piepsz A et al (2006) Primary vesicoureteral reflux detected in neonates with a history of fetal renal pelvis dilatation: a prospective clinical and imaging study. J Pediatr 148:222
Psooy K, Pike J (2009) Investigation and management of antenatally detected hydronephrosis. Can Urol Assoc J 3:69
Walsh TJ, Hsieh S, Grady R et al (2007) Antenatal hydronephrosis and the risk of pyelonephritis hospitalization during the first year of life. Urology 69:970
Coelho GM, Bouzada MC, Lemos GS et al (2008) Risk factors for urinary tract infection in children with prenatal renal pelvic dilatation. J Urol 179:284
Lee JH, Choi HS, Kim JK et al (2008) Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol 179:1524
Estrada CR, Peters CA, Retik AB et al (2009) Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis—should voiding cystourethrography be performed in cases of postnatally persistent grade II hydronephrosis? J Urol 181:801
Roth CC, Hubanks JM, Bright BC et al (2009) Occurrence of urinary tract infection in children with significant upper urinary tract obstruction. Urology 73:74
Circumcision policy statement (2012) American Academy of Pediatrics Task Force on Circumcision. Pediatrics 130:585–586
Mukherjee S, Joshi A, Carroll D et al (2009) What is the effect of circumcision on risk of urinary tract infection in boys with posterior urethral valves? J Pediatr Surg 44:417
Herndon CD, McKenna PH, Kolon TF et al (1999) A multicenter outcomes analysis of patients with neonatal reflux presenting with prenatal hydronephrosis. J Urol 162:1203
Ismaili K, Avni FE, Wissing KM et al (2004) Long-term clinical outcome of infants with mild and moderate fetal pyelectasis: validation of neonatal ultrasound as a screening tool to detect significant nephrouropathies. J Pediatr 144:759
Ismaili K, Avni FE, Alexander M et al (2005) Routine voiding cystourethrography is of no value in neonates with unilateral multicystic dysplastic kidney. J Pediatr 146:759
Roth JA, Diamond DA (2001) Prenatal hydronephrosis. Curr Opin Pediatr 13:138
Chung S, Majd M, Rushton HG et al (1993) Diuretic renography in the evaluation of neonatal hydronephrosis: is it reliable? J Urol 150:765
Ross SS, Kardos S, Krill A et al (2011) Observation of infants with SFU grades 3–4 hydronephrosis: worsening drainage with serial diuresis renography indicates surgical intervention and helps prevent loss of renal function. J Pediatr Urol 7:266–271
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davenport, M.T., Merguerian, P.A. & Koyle, M. Antenatally diagnosed hydronephrosis: current postnatal management. Pediatr Surg Int 29, 207–214 (2013). https://doi.org/10.1007/s00383-012-3258-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3258-4