Abstract
Purpose
While the endocrine action of the active metabolite 1,25-dihydroxyvitamin D (VtD) has been well characterized in relation to the maintenance of plasma calcium and phosphate homeostasis through regulation of intestinal absorption, recent research has focused on its autocrine and/or paracrine activities. Such activities have been best characterized in intestine, where VtD regulates cell differentiation and maturation. The purpose of this study was to evaluate the effect of VtD on enterocyte turnover in a rat model of short bowel syndrome (SBS).
Methods
Male rats were divided into four groups: sham rats underwent bowel transection, sham-VtD rats underwent bowel transection and were treated oral VtD, SBS rats underwent a 75 % bowel resection, and SBS-VtD rats underwent bowel resection and were treated with VtD. Parameters of intestinal adaptation, enterocyte proliferation and apoptosis were determined at sacrifice. Illumina’s digital gene expression (DGE) analysis was used to determine VtD pathway-related gene expression profiling. VtD receptor (VDR) and its promoter, Bax and Bcl-2 mRNA expression were determined using real-time PCR. Western blotting was used to determine p-ERK, Bax and β-catenin protein levels.
Results
From the total of 20,000 probes, 11 genes related to VtD signaling were investigated. Of these genes, five were found to be up-regulated in SBS versus sham animals with a relative change in gene expression level of 20 %, five remained unchanged, and one was down-regulated. VtD treatment in sham and SBS rats resulted in significant up-regulation of the VDR gene and its promoter’s expression. SBS-VtD rats demonstrated a significant increase in all intestinal mucosal parameters compared to SBS animals. A significant increase in cell proliferation in SBS-VtD rats was accompanied by increased β-catenin protein levels. A significant decrease in cell apoptosis in this group was correlated with lower Bax/Bcl-2 mRNA and protein levels.
Conclusion
In a rat model of SBS, dietary supplementation with VtD stimulates enterocyte turnover, which correlates with up-regulated VtD receptor expression in the remaining small intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short bowel syndrome (SBS) is a clinical condition defined as a state of malnutrition and malabsorption of nutrients and micronutrients, resulting from the surgical removal of small bowel and decrease in absorptive surface area [1, 2]. Despite progress in critical care and long-term nutritional support, mortality and morbidity in patients with SBS remain high [3]. The most important factor contributing to outcome in SBS patients is the capacity of the intestinal remnants to undergo an adaptation process [4]. Intestinal adaptation begins within 24–48 h post-bowel resection, represented by structural and functional changes. Morphological changes include elongation and dilatation of the remaining bowel, lengthening of villi and deepening of crypts, resulting in increased absorptive surface area. Functional changes result in increased absorptive capacity of individual enterocytes.
Various nutrients, hormones and peptide growth factors stimulate intestinal adaptation, with luminal nutrients being particularly important [5]. Prolonged “bowel rest” along with total parenteral nutrition may impair intestinal adaptation and patients’ ability to achieve nutritional autonomy. Therefore, once patients stabilize, slow introduction of enteral feeding is indicated. Dietary constituents may have profound positive or negative effects on intestinal adaptation and should be considered in developing an overall treatment plan for patients with SBS [6, 7]. The mechanism whereby nutrients induce this adaptation is unknown. It is likely that nutritional factors work through a number of mechanisms, including stimulation of mucosal hyperplasia by direct contact with epithelial cells, stimulation of trophic gastrointestinal hormone secretion and stimulation of the production of trophic pancreaticobiliary secretions.
The absorption of many nutrients is impaired following resection, with lipid absorption generally considered most vulnerable. The combined loss of absorptive surface area and compromised enterohepatic circulation, decreased bile acid pool and decreased pancreatic lipase secretion results in steatorrhea and inefficient lipid absorption [8, 9]. Fat malabsorption is associated with poor absorption of fat-soluble vitamins (A, D, E, and K) that are primarily stored in the liver and adipose tissues. Therefore, fat-soluble (as well as water-soluble) vitamins should be accounted for in the SBS diet. Serum 25(OH) vitamin D levels can be normalized by giving 50,000 IU of oral vitamin D every other day until normalization of plasma levels occurs [10].
Although the endocrine action of the active metabolite 1,25-dihydroxyvitamin D (VtD) has been well characterized in relation to the maintenance of plasma calcium and phosphate homeostasis through regulation of intestinal absorption, recent evidence suggests that VtD exerts autocrine and/or paracrine activities. Such activities have been best characterized in intestine, where VtD regulates cell differentiation and maturation. [11]. VtD mediates its biological effects by binding to VtD receptors (VDRs), which are principally located in target cell nuclei. Calcitriol binding allows the VDR to act as a transcription factor which modulates gene expression of transport proteins such as TRPV6 and calbindin, which in turn influence intestinal calcium absorption [12]. The VDR belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDRs are expressed in most organs, including the brain, heart, skin, gonads, prostate, breast and intestine [13]. VDR activation in intestine, bone, kidney, and parathyroid gland cells aids in the maintenance of calcium and phosphorus levels in blood and in the bone [14].
The purpose of this study was to evaluate the effects of VtD on intestinal regrowth following massive small bowel resection in a rat model and to clarify the mechanisms by which VtD influences mucosal hyperplasia, enterocyte proliferation and cell death via apoptosis. Additionally, this study aimed to determine whether the effects of VtD on enterocyte turnover correlate with VDR expression throughout the gastrointestinal tract.
Materials and methods
Animals
Surgical procedures and animal care were conducted in compliance with the guidelines established by the “Guide for the Care and Use of Laboratory Animals”, Rappaport Faculty of Medicine, Technion (Haifa, Israel). Adult male Sprague–Dawley rats weighing approximately 250–260 g were maintained in a 12-h light–dark cycle (6 a.m.—lights on, 6 p.m.—lights off) and were allowed free access to water and standard rat chow for 5–7 days to acclimatize to the housing conditions.
Experimental design
In the first experiment, the role of VtD signaling following massive small bowel resection was investigated. Animals were randomly divided into two groups of eight rats each. Group A (sham) rats underwent bowel transection and re-anastomosis; Group B (SBS) rats underwent 75 % bowel resection. Illumina’s digital gene expression (DGE) analysis using Illumina Rat Quad BeadChip was used to determine VtD-related gene expression profiling in jejunum and ileum [15].
In the second experiment, rats were randomly divided into four groups. Group A (sham) rats (n = 8) underwent bowel transection; Group B (sham-VtD) rats (n = 8) underwent bowel transection and were treated with VtD by gavage at a dose of 100 mg/kg from day 4 to day 14; Group C (SBS) rats (n = 8) underwent 75 % bowel resection; Group D (SBS-VtD) rats (n = 8) underwent bowel resection and were treated with VtD similar to Group B. Dosage of VtD was chosen in accordance with previously described studies [16].
Surgical procedure
Following an overnight fast, the rats were anesthetized with an intraperitoneal injection of pentobarbital (45 mg/kg) and the abdomen opened through a midline incision. Sham rats underwent laparotomy, bowel transection and re-anastomosis at 10 cm proximal to the ileocecal junction. In SBS rats, the proximal 75 % of intestine was resected. The proximal jejunum was anastomosed to the remaining ileum in continuity using interrupted 5-0 silk sutures.
All rats were killed on the 15th postoperative day. The small intestine from the pylorus to the ileocecal valve was removed and divided at the anastomosis. Portions of intestine 1 cm on either side of the anastomosis were discarded because of the surgically induced hyperplasia occurring in the peri-anastomotic region. The intestine was split on the antimesenteric border, washed with cold saline, dried, and each segment was weighed. The mucosa was scraped from the underlying tissue with a glass slide and mucosal samples were subsequently homogenized with TRIzol reagent.
Genome sequencing library preparations
Microarray expression profiling was performed in the Genomics Core Facility (BioRap Technologies, Rappaport Research Institute, Technion). Total RNA was isolated using a TRIzol (Invitrogen, Carlsbad, CA, USA) extraction protocol. The RNA was amplified into cRNA and biotinylated by in vitro transcription using the TargetAmp Nano-g Biotin-aRNA labeling kit for the Illumina system (Epicentre Biotechnologies) according to manufacturer’s protocol. Biotinylated cRNA was purified, fragmented, and subsequently hybridized to an Illumina RatRef-12 Expression BeadChip V1 for genome-wide expression analysis containing 21,910 probes selected primarily from the NCBI RefSeq database (Release 16) according to the Direct Hybridization assay (Illumina Inc.). The hybridized chip capable of querying 12 samples in parallel was stained with streptavidin-Cy3 (AmershamTM) and scanned with an Illumina BeadArray 500GX Reader. The scanned images were imported into GenomeStudio (Illumina Inc.) for extraction and quality control, generating an output file for statistical analysis. Pathway analysis was performed using Ingenuity Pathway Analysis (IPA) to identify canonical (i.e., cell signaling and metabolic) pathways and gene–gene interaction networks potentially involved in SBS within our dataset [17].
Histology
Intestinal samples from the proximal jejunum and distal ileum were fixed in 10 % formalin, dehydrated in progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin wax. Deparaffinized 5 μm sections were stained with hematoxylin and eosin. Villus height and crypt depth were measured using a graded eye piece at ten times magnification by a pathologist blinded as to tissue origin.
Enterocyte proliferation and apoptosis
Portions of the proximal jejunum and distal ileum were taken as described above. Crypt cell proliferation was assessed using 5-bromodeoxyuridine (5-BrdU). Standard BrdU labeling reagent (Zymed Laboratories, Inc., San Francisco, CA, USA) was injected intraperitoneally at a concentration of 1 ml/100 g body weight 2 h prior to killing. Tissue slices (5 μm) were stained with a biotinylated monoclonal anti-BrdU antibody system provided in kit form (Zymed Laboratories, Inc., San Francisco, CA, USA). The index of proliferation was determined as the ratio of crypt cells staining positively for BrdU per 10 crypts. Additional 5 μm thick sections were prepared to establish the degree of enterocyte apoptosis. Immunohistochemistry for Caspase-3 (Caspase-3 cleaved polyclonal antibody, dilution 1:100, CP229B, Biocare Medical, LLC, CA 94520, USA) was performed for identification of apoptotic cells using a combination of the streptavidin–biotin–peroxidase method and microwave antigen retrieval on formalin-fixed, paraffin-embedded tissues according to the manufacturer’s protocols. A qualified pathologist blinded as to the source of intestinal tissue performed all measurements.
Real-time PCR
RNA was isolated using TRIzol (Invitrogen) reagent according to the manufacturer’s instructions, and quantification of RNA was performed using 260/280 nm spectrophotometry. The method was extended using reverse transcriptase (PrimeScript RT reagent Kit Takara, Japan) to convert 500 ng of total RNA into complementary DNA (cDNA) which was then amplified by PCR-Thermal Cycler (2720 Thermal Cycler, ABI, Israel). Gene Expression of VDR, VDR promoter, Bax and Bcl-2 mRNA was determined by quantitative real-time PCR ABI-PRISM 7000 (applied Biosystems, Foster City, CA, USA) on cDNA samples using Cyber Green Master Mix (Takara, Japan) with the exception of template and primers.
Western blotting
Tissue was homogenized in RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 % NP-40, 2 mM EDTA, supplemented with a cocktail of protease (Roche Diagnostic) and phosphatase cocktail inhibitors (Sigma). Protein concentrations were determined by Bradford reagent according to the manufacturer’s instructions. Samples containing equal amounts of total protein (30 μg) were resolved by SDS-PAGE under reducing conditions. After electrophoresis, proteins were transferred to PVDF membrane and probed with various primary anti-Bax antibody (1:2,000 dilution, sc-493), anti-phospho-ERK antibody (1:2,500 dilution, sc-7383), anti-β-catenin antibody (1:500 dilution, sc-8312) and anti-ERK2 antibody (1:2,000 dilution, Cell Signaling #9108). Horseradish peroxidase-conjugated secondary antibody was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA) and an enhanced chemiluminescent substrate from Biological Industries (Kibbutz Beth HaEmek, Israel). The optical density of the specific protein bands was quantified using a densitometer (Vilber Lourmat, Lyon, France).
Statistical analysis
The data are expressed as the mean ± SEM. Statistical analysis of adaptation parameters, enterocyte proliferation, and apoptosis was performed using the non-parametric Kruskal–Wallis ANOVA test, followed by the corrected Mann–Whitney test, with p < 0.05 considered statistically significant.
Results
First experiment (microarray expression profiling)
165 genes were differentially expressed in rat intestine after massive small bowel resection when compared to controls (fold change >1.2, p < 0.001). Functional clustering analysis revealed increased expression in signaling pathways involved in tryptophan, ascorbate and aldarate metabolism, biosynthesis of steroids, propanoate metabolism, glycine, serine and threonine metabolism, and fatty acid metabolism. From the total amount of 20,000 probes, 11 genes related to VtD signaling were investigated (Table 1). From these genes, five genes were up-regulated in jejunum and seven genes were up-regulated in ileum in SBS rats compared to sham rats. Five genes in jejunum and two genes in ileum remained unchanged. The VDR gene was up-regulated in jejunum and down-regulated in ileum. Moreover, cluster analysis based on all genes represented on the microarray chip showed a clear differentiation. The most significant increase was shown in Cyp1a1 (cytochrome P450, family 1, subfamily a, polypeptide 1) and Cyp51 (cytochrome P450, family 51); a threefold and twofold increase, respectively. A significant 20–70 % up-regulation was also observed in the levels of STAT1, STAT 2 and STAT 3 (signal transducer and activator of transcription).
Second experiment
Body weight changes
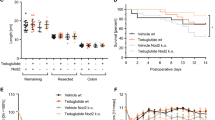
Massive small bowel resection resulted in a significant decrease in SBS rat versus sham rat body weight (102 ± 3 and 119 ± 3 % initial weight, p < 0.001; Fig. 1). Although VtD treatment in SBS animals resulted in a trend toward increase in final body weight (compared to SBS animals), this trend was not statistically significant.
Parameters of intestinal adaptation
SBS rats demonstrated a significant increase in overall bowel and mucosal weight in jejunum (fourfold increase, p < 0.001) and in ileum (twofold increase, p < 0.001) (Fig. 2), villus height and crypt depth in jejunum (478 ± 37 vs. 385 ± 17 μm and 201 ± 8 vs. 139 ± 5 μm, respectively, p < 0.05) and ileum (448 ± 13 vs. 291 ± 29 μm and 167 ± 14 vs. 135 ± 7 μm, respectively, p < 0.05) (Fig. 3) compared to sham animals. SBS-VtD rats demonstrated an additional increase in overall bowel weight in jejunum and ileum (13 %, p < 0.05), mucosal weight in jejunum and ileum (37 and 16 %, respectively, p < 0.05) (Fig. 2), villus height in jejunum (567 ± 11 vs. 478 ± 37 μm, p < 0.05) and ileum (508 ± 36 vs. 448 ± 13 μm, p < 0.05) and crypt depth in ileum (209 ± 12 vs. 167 ± 14 μm, p < 0.05) (Fig. 3) compared to SBS rats.
Enterocyte proliferation and apoptosis
Treatment of sham animals with VtD resulted in a significant increase in cell proliferation rates in jejunum (153 ± 4 vs. 129 ± 3 BrdU positive cells/10 crypts, p < 0.001) and ileum (153 ± 3 vs. 126 ± 3 BrdU positive cells/10 crypts, p < 0.001) compared to sham animals, and in a trend toward concomitant increase in cell apoptosis in jejunum. However, this trend was not statistically significant (Fig. 4). SBS rats demonstrated a significant increase in cell proliferation in jejunum (31 %, p < 0.001) and ileum (29 %, p < 0.001) and concomitant increase in cell apoptosis in jejunum (threefold increase, p < 0.001) and ileum (twofold increase, p < 0.001) compared to sham rats. Treatment of SBS animals with VtD resulted in an additional increase in cell proliferation rate in jejunum (16 %, p < 0.05) and ileum (19 %, p < 0.001) compared to SBS rats. SBS-VtD rats also demonstrated a significant decrease in cell apoptosis in jejunum and ileum (twofold decrease, p < 0.001) compared to SBS animals.
Effect of bowel resection and exogenous VtD on crypt cell proliferation and enterocyte apoptosis. 5-BrdU incorporation into proliferating jejunal and ileal crypt cells was detected with a goat anti-BrdU antibody, and immunochemistry for caspase-3 was used to determine enterocyte apoptosis. Values are mean ± SEM. SBS short bowel syndrome; VtD vitamin D. *p < 0.05 SBS-VtD and SBS rats versus sham rats, † p < 0.05 SBS-VtD versus SBS rats
Real-time PCR
The effect of bowel resection on VDR, VDR Promoter, Bax and Bcl-2 mRNA throughout the gastrointestinal tract was determined using real-time PCR (Fig. 5). As expected, treatment of sham animals with VtD resulted in a significant up-regulation of VDR and its promoter in both jejunum and ileum. Massive small bowel resection did not significantly change VDR and its promoter mRNA expression compared to control animals. Treatment of SBS animals with VtD resulted in a trend toward increase in receptor and promoter expression in ileum; however, this trend was not statistically significant. Massive small bowel resection (SBS) resulted in a significant increase in Bax mRNA expression in jejunum (fourfold increase, p < 0.05) and ileum (threefold increase, p < 0.05) and a significant twofold decrease in Bcl-2 mRNA level in ileum (p < 0.05) compared to control animals. The Bax to Bcl-2 ratio increased significantly in SBS rats compared to control animals in both jejunum (fivefold increase, p < 0.05) and ileum (fourfold increase, p < 0.05), which was in agreement with elevated cell apoptosis in this experimental group. VtD treatment resulted in an eightfold decrease in Bax mRNA expression in jejunum and fourfold decrease in Bax mRNA expression in ileum compared to SBS rats. Interestingly, Bcl-2 mRNA levels also decreased in this group; however, this decrease was less marked compared to changes in Bax mRNA levels. Correspondingly, Bax/Bcl-2 ratio decreased in jejunum (threefold decrease) and ileum (twofold decrease) in SBS-VtD rats compared to SBS animals, suggesting increased enterocyte survival.
Effect of bowel resection and treatment with VtD on VDR, VDR Promoter, Bax and Bcl-2 mRNA throughout the gastrointestinal tract (using real-time PCR). Values are mean ± SEM. SBS short bowel syndrome; VtD vitamin D, VDR vitamin D receptor. *p < 0.05 SBS-VtD and SBS rats versus sham rats, † p < 0.05 SBS-VtD versus SBS rats
Western blot
Elevated cell proliferation rates in SBS (vs. sham) animals were accompanied by elevated β-catenin protein levels in jejunum (twofold increase, p < 0.05) and ileum (threefold increase, p < 0.05) (Fig. 6). Treatment of both sham and SBS animals with VtD resulted in a significant up-regulation of β-catenin protein levels compared to non-treated animals. A significant increase in cell apoptosis in SBS (vs. sham) rats was accompanied by a significant increase in pro-apoptotic Bax gene expression in jejunum (twofold increase, p < 0.05) and ileum (fourfold increase, p < 0.05). Treatment with VtD led to significant threefold down-regulation in Bax mRNA expression in both jejunum and ileum compared to SBS animals (p < 0.05), which was in agreement with decreased cell apoptosis.
Discussion
Previous studies in VDR knockout mice indicated that the major role of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is intestinal calcium transport [18, 19]. However, the exact mechanisms of the non-classical actions of vitamin D remain to be defined. 1,25(OH)2D3 has been reported to inhibit the proliferation of a number of malignant cells and to have immunosuppressive effects [20]. Enhanced VDR transcription and up-regulated p21 (which functions as a regulator of cell cycle progression) were found to correlate with the anti-proliferative effects of 1,25(OH)2D3 in several cancer cells [21].
Several experimental studies have described autocrine and paracrine activities of VtD in intestine, where it regulates cell differentiation and maturation. Therefore, in the current study we hypothesized that VtD signaling may be involved in intestinal tissue re-growth after massive bowel resection and that exogenous VtD may have a positive effect in stimulating intestinal adaptation.
In the first experiment, a role of VtD signaling following massive small bowel resection was investigated. Microarray expression profiling demonstrated that most of the VtD signaling-related genes were up-regulated in resected rats compared to control animals, suggesting an important role of this signaling pathway in the post-resection intestinal response. Five genes were significantly up-regulated following bowel resection. The most significant increase was shown in Cyp1a1 (cytochrome P450, family 1, subfamily a, polypeptide 1; threefold increase) and Cyp51 (cytochrome P450, family 51; twofold increase). Cytochrome P450 (CYP) proteins generally catalyze mono-oxygenations and are found in all biological kingdoms. Cytochrome P450 1A1 (CYP1A1) is a member of the CYP family that participates in the metabolism of xenobiotics and endogenous compounds, particularly polycyclic aromatic hydrocarbons such as benzo[a]pyrene (BaP) [22]. BaP activates the aryl hydrocarbon (AHR) and induces the expression of genes involved in xenobiotic metabolism, including CYP1A1. CYP1A1 is involved not only in BaP detoxification but also in metabolic activation, which results in DNA adduct formation. VDR belongs to the NR1I subfamily of the nuclear receptor superfamily, which also regulates expression of xenobiotic metabolism genes. Investigation of the cross-talk between AHR and VDR signaling pathways found that 1α,25-dihydroxyvitamin D3, a potent physiological VDR agonist, enhanced BaP-induced transcription of CYP1A1 in human monocytic U937 cells and THP-1 cells, breast cancer cells and kidney epithelium-derived cells [23]. Several experiments have shown that cytochrome P450 enzymes in intestinal tissue play a dominant role in target tissue metabolic activation of xenobiotic compounds. They also determine drug efficacy and influence the tissue burden of foreign chemicals and bioavailability of therapeutic agents [24].
A significant 20–70 % up-regulation was observed in the current study also in the levels of STAT1, STAT 2 and STAT 3 (signal transducer and activator of transcription). Cellular responses to dozens of cytokines and growth factors are mediated by the evolutionarily conserved Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway. These responses include proliferation, differentiation, migration, apoptosis, and cell survival [25]. Recent evidence suggests that VtD modulates JAK-STAT signaling pathway [26]. JAK/STAT signaling is essential for numerous developmental and homeostatic processes, including hematopoiesis, immune cell development, stem cell maintenance, organismal growth, and mammary gland development. Although the canonical JAK/STAT pathway is simple and direct, pathway components regulate or are regulated by members of other signaling pathways, including those involving the ERK MAP kinase, PI 3-kinase (PI3K), β-catenin and others [25].
Next, we investigated whether exogenous VtD might stimulate intestinal re-growth following massive small bowel resection. Our results show that massive bowel resection in rats results in apparent stimulation of structural intestinal adaptation. This is evident as increased bowel and mucosal weight and villus height and crypt depth, reflecting a promoted adaptive response. Comparison of morphometric parameters revealed intestinal hyperplasia to be a predominant feature of mucosal adaptation. Compared with the sham-transected group, villus height and crypt depth were significantly increased in SBS animals, suggesting increased absorptive surface area. The rate of enterocyte proliferation increased in SBS rats compared to sham animals and was accompanied by elevated β-catenin protein levels in jejunum (twofold increase) and ileum (threefold increase). The role of β-catenin in stimulating stem cell activity after bowel resection has been discussed in several recent experiments [27, 28]. Elevated cell proliferation in the current study was accompanied by increased rates of enterocyte apoptosis, suggesting accelerated cell turnover. An increased cell apoptosis may be considered the mechanism that counterbalances the increased enterocyte proliferation to reach a new homeostatic set during intestinal adaptation. In addition, increased apoptosis promotes disposal of genetically aberrant stem cells and prevents tumorigenesis [29]. Bax protein level was upregulated, while Bcl-2 mRNA was down-regulated during intestinal adaptation. As a result, the Bax/Bcl-2 ratio increased in SBS rats, which correlates with the enhanced enterocyte apoptosis in this group. These changes were in agreement with previous findings which demonstrated an important role of Bax in enhancing programmed cell death after small bowel resection [30].
Administration of VtD in the current study significantly enhanced structural intestinal adaptation. VtD-treated rats demonstrated an additional increase in bowel and mucosal weight as well as an increased villus height and crypt depth, which are the result of increased proliferation and accelerated migration along the villus, and are a marker of increased absorptive surface area. The observed increase in enterocyte proliferation rate may be considered as a main mechanism responsible for compensatory hyperplasia and is responsible for increased intestinal cell mass. The observed stimulation of cell proliferation was in agreement with elevated β-catenin protein. Much evidence is available to show that VtD not only regulates the synthesis of growth factors and cytokines, but also modulates growth-factor signaling [31]. Chakrabarty et al. [32] have recently observed that exposure of human colon tumor cells to extracellular calcium suppressed β-catenin transcriptional activation and promoted E-cadherin expression. Interesting findings by Palmer et al. indicate that 1,25(OH)2D3 increased transcription of the gene encoding E-cadherin and promoted translocation of β-catenin from the nucleus to the plasma membrane. Activated VDR competed with TCF transcription factors for β-catenin binding and, therefore, interfered with inappropriate β-catenin-mediated transcriptional activity [33].
Enterocyte apoptosis decreased significantly in SBS-VtD rats compared to SBS animals, which was consistent with decreased expression of Bax mRNA and protein levels. Recent evidence suggests that calcium and VtD may have stronger effects on cell differentiation and apoptosis than on proliferation; and that even relatively low-dose vitamin D may have greater effects on colorectal epithelial cell differentiation and apoptosis than high-dose calcium alone or in combination with low-dose vitamin D [34, 35]. Our observations are contrary to the recent report of pro-apoptotic effects of calcium and VtD in different cancer cells. There is a large body of evidence which indicates that VtD exerts an anticancer action by inducing apoptosis in various transformed cells, including colon cancer cells [36, 37]. Diaz and colleagues [38] have shown that 1,25(OH)2D3 induces apoptosis in human colon adenoma and carcinoma cell lines that was associated with up-regulation of expression of the pro-apoptotic protein Bax and BAK (Bcl-2 antagonists).
Next, we investigated whether the effects of exogenous VtD on enterocyte turnover (proliferation and apoptosis) correlate with VDR expression throughout the gastrointestinal tract. We have shown that the proliferative and anti-apoptotic effect of VtD on enterocyte turnover correlated with elevated VDR expression following VtD administration in both sham and resected animals; however, in control rats, this change was much more significant. The up-regulation of VDR expression together with elevated β-catenin levels emphasizes the possible effect of VtD in the control of stem cell activity after VtD administration.
In conclusion, (1) bowel resection in a rat model results in activation of VtD signaling; (2) exogenous VtD stimulates intestinal re-growth after massive small bowel resection; (3) the stimulating effect of VtD on enterocyte turnover (proliferation and apoptosis) correlates with VDR expression throughout the gastrointestinal tract.
References
Biller JA (1987) Short bowel syndrome. In: Grand RI, Sutphen JL, Dietz WH (eds) Pediatric nutrition. Theory and practice. Butterworth Publishers, Stoneham, pp 481–487
Booth IW, Lander AD (1998) Short bowel syndrome. Baillieres Clin Gastroenterol 12:739–772
Coran AG, Spivak D, Teitelbaum DH (1999) An analysis of the morbidity and mortality of short bowel syndrome in the pediatric age group. Eur J Pediatr Surg 9:228–230
O’Brien DP, Nelson LA, Huang FS, Warner BW (2001) Intestinal adaptation: structure, function, and regulation. Semin Pediatr Surg 10:56–64
Levine GM, Deren JJ, Yezdimir E (1976) Small bowel resection: oral intake is the stimulus for hyperplasia. Am J Dig Dis 21:542–546
Lentze MJ (1989) Intestinal adaptation in short-bowel syndrome. Eur J Pediatr 148:294–299
Park JHY, Grandjean CJ, Hart MH, Vanderhoof JA (1989) Effects of dietary linoleic acid on mucosal adaptation after small bowel resection. Digestion 44:57–65
Vanderhoof JA, Burkley KT, Antonson KT (1983) Potential for mucosal adaptation following massive small bowel resection in 3-week-old versus 8-week-old rats. J Pediatr Gastroenterol Nutr 2:672–676
Chiba T, Ohi R (1998) Do we still need to collect stool? Evaluation of visualized fatty acid absorption: experimental studies using rats. J Parenter Enteral Nutr 22:22–26
Sundaram A, Koutkia P, Apovian C (2002) Nutritional management of short bowel syndrome in adults. J Clin Gastroenterol 34:207–220
Vandewalle B, Wattez N, Lefebvre J (1995) Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT-29 cells: possible implication of intracellular calcium. Cancer Lett 97:99–106
Bouillon R, Van Cromphaut S, Carmeliet G (2003) Intestinal calcium absorption: molecular vitamin D mediated mechanisms. J Cell Biochem 88:332–339
Covic A, Apetrii M (2011) Vitamin D receptor activation: clinical outcomes. Contrib Nephrol 171:166–171
Warnock DG (2011) Vitamin D receptor activation: implications for daily practice. Contrib Nephrol 171:172–180
Du P, Kibbe WA, Lin SM (2008) Lumi: a pipeline for processing Illumina microarray. Bioinformatics 24:1547–1548
Harada S, Mizoguchi T, Kobayashi Y, Nakamichi Y, Takeda S, Sakai S, Takahashi F, Saito H, Yasuda H, Udagawa N, Suda T, Takahashi N (2012) Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res 27:461–473
Lin SM, Du P, Huber W, Kibbe WA (2008) Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res 36:e11–e17
Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB (1997) Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S (1997) Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396
Dhawan P, Wieder R, Christakos S (2009) CCAAT enhancer binding protein alpha is a molecular target of 1,25-dihydroxyvitamin D3 in MCF-7 breast cancer cells. J Biol Chem 284:3086–3095
Christakos S, Dhawan P, Ajibade D, Benn BS, Feng J, Joshi SS (2010) Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J Steroid Biochem Mol Biol 121:183–187
Guengerich FP, Shimada T (1998) Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res 400:201–213
Matsunawa M, Akagi D, Uno S, Endo-Umeda K, Yamada S, Ikeda K, Makisima M (2012) Vitamin D receptor activation enhances Benzo[a]pyrene metabolism via CYP1A1 expression in macrophages. Drug Metab Dispos (in press)
Diing X, Kaminsky LS (2003) Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol 43:149–173
Harrison DA (2012) The Jak/STAT pathway. Cold Spring Harb Perspect Biol 4:a011205
Muthian G, Raikwar HP, Rajasingh J, Bright JJ (2006) 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res 83:1299–1309
Taylor JA, Bernabe KQ, Guo J, Warner BW (2007) Epidermal growth factor receptor-directed enterocyte proliferation does not induce Wnt pathway transcription. J Pediatr Surg 42:981–986
Juno RJ, Knott AW, Profitt SA, Jarboe MD, Zhang Y, Erwin CR, Warner BW (2004) Preventing enterocyte apoptosis after massive small bowel resection does not enhance adaptation of the intestinal mucosa. J Pediatr Surg 39:907–911
Wildhaber BE, Yang H, Coran AG, Teitelbaum DH (2003) Gene alteration of intestinal intraepithelial lymphocytes in response to massive small bowel resection. Pediatr Surg Int 19:310–315
Haxhija EQ, Yang H, Spencer AU, Sun X, Teitelbaum DH (2006) Influence of the site of small bowel resection on intestinal epithelial cell apoptosis. Pediatr Surg Int 22:37–42
Gurlek A, Pittelkow MR, Kumar R (2002) Modulation of growth factor/cytokine synthesis and signaling by 1,25-dihydroxyvitamin D3: implications in cell growth and differentiation. Endocrine Rev 23:763–786
Chakrabarty S, Radjendirane V, Appelman H, Varani J (2003) Extracellular calcium and calcium-sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of -catenin/TCF activation. Cancer Res 63:67–71
Pálmer HG, Sánchez-Carbayo M, Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C, Muñoz A (2001) Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of -catenin signaling. J Cell Biol 154:369–388
Bostick RM, Fosdick L, Wood JR, Grambsch P, Grandits GA, Lillemoe TJ, Louis TA, Potter JD (1995) Calcium and colorectal epithelial cell proliferation in sporadic adenoma patients: a randomized, double-blinded, placebo-controlled clinical trial. J Natl Cancer Inst 87:1307–1315
Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, Daniel CR, Rutherford RE, Woodard JJ (2009) Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res 2:213–223
Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvälä H, Vienonen A, Tuohimaa P (2002) Antiproliferative action of vitamin D. Vitam Horm 64:357–405
Vandewalle B, Wattez N, Lefebvre J (1995) Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT-29 cells: possible implication of intracellular calcium. Cancer Lett 97:99–106
Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A (2000) Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res 60(8):2304–2312
Acknowledgments
This work was supported by the Atkins Medical Research Fund (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadjittofi, C., Coran, A.G., Mogilner, J.G. et al. Dietary supplementation with vitamin D stimulates intestinal epithelial cell turnover after massive small bowel resection in rats. Pediatr Surg Int 29, 41–50 (2013). https://doi.org/10.1007/s00383-012-3205-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3205-4