Abstract
Objective
The role of preoperative contrast-enhanced computerized tomography (CT) of chest with three-dimensional (3D) reconstructions was evaluated in neonates with esophageal atresia and tracheoesophageal fistula.
Methods
This was a prospective study which investigated 30 cases of esophageal atresia with tracheoesophageal fistula. All patients were evaluated preoperatively with contrast-enhanced spiral CT using a low-dose CT protocol. 3D CT reconstruction images were evaluated for the type of esophageal atresia, the distance between the upper and lower esophageal pouches, origin, level and position of the fistula, and the presence or absence of any other cardiac, pulmonary or mediastinal lesions and the findings were correlated with the findings at surgery. The radiation dose for each patient was calculated using the formula—Effective dose (E) = DLP × (E/DLP)age.
Results
All the 30 cases had type-C esophageal atresia with tracheoesophageal fistula as per Gross classification. The exact site of the fistula could be identified only in 26 (80 %) cases. The mean gap between the upper pouch and lower fistula was 0.95 ± 0.57 cm (range 0.2–2.8 cm) on CT scan and 1.38 ± 0.61 cm (range 0.5–3.2 cm) at surgery. On statistical analysis, the correlation was found to be significant (p < 0.0001). In addition, lung pathology (consolidation), cardiac pathology and vertebral anomaly were also detected on CT scan in some cases. The mean radiation dose for the neonates who underwent CT chest was calculated to be 1.79 mSv which is significantly high.
Conclusion
Though preoperative CT scan of chest has many advantages, it involves significant exposure to ionizing radiation and risk of radiation-induced cancer in the future. Additionally in 20 % of cases, the fistula could not be located on CT scan. The most common variety of esophageal atresia and tracheoesophageal fistula is Gross type C (86 %) that has low to intermediate gap (97 %) and can be anastomosed primarily. Thus, CT scan can provide good anatomical delineation, but may not help in surgical decision making. Hence, performing CT in these cases would unnecessarily expose the neonates to ionizing radiation. Therefore, there is no role for CT scan in the routine preoperative assessment of EA with distal TEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal atresia (EA) with tracheoesophageal fistula (TEF) is one of the most common correctable congenital anomalies encountered in pediatric surgery [1]. Of all the cases of EA with TEF, EA with distal TEF is the most common anomaly (86 %). The reported incidence of EA with proximal TEF is only 0.4–3 %. A few recent studies have found the incidence of proximal fistula to be between 5 and 7.7 %, arguing that most of these are missed at initial surgery [2, 3]. Proper preoperative assessment helps in prognostication and proper surgical care. Apart from the clinical assessment, chest radiograph with a stiff orogastric tube has been the preferred investigation. Assessment of the interpouch gap is a crucial factor in risk stratification and prognosis. Preoperative endoscopy and bronchoscopy have been advocated by many as an adjunct to these studies. Apart from being invasive, these investigations do not help in accurate assessment of gap [19–21]. The advent of computerized tomography (CT) has revolutionized diagnostic imaging and many authors have advocated the use of CT scan in preoperative evaluation of EA in TEF patients, as it is a quick, noninvasive procedure that precisely delineates the anatomy of TEF, accurately assesses the interpouch gap and delineates the spatial relationships of the two esophageal pouches and the TEF with the help of 3D images [24–28]. Preoperative CT of chest has also been previously reported as a useful investigation to diagnose proximal and H-type fistula [4].
Although CT scan has been advised as a good adjunctive study in preoperative evaluation of EA with TEF, issues with radiation exposure from CT scanning have never been highlighted in previously described series. So, the present study was conducted for defining the role of preoperative 3D CT reconstruction in patients with EA with TEF. We also calculated the radiation dose, so as to find out whether the information from the 3D CT reconstruction was of benefit to the patient and surgeon in view of associated radiation exposure.
Materials and methods
This prospective study was conducted between September 2009 and May 2011, at the Departments of Pediatric Surgery and Radio Diagnosis and Imaging, Advanced Pediatric Center, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. The study protocol was analyzed and approved by the ethical committee of PGIMER. A separate consent paper was prepared for the parents, with detailed information regarding purpose of the study, procedure details, X-ray radiation risk and CT scan risks.
Thirty cases of EA with TEF, admitted in the neonatal surgical intensive care unit (NSICU), were randomly selected to be included in the study. Clinical diagnosis of EA with TEF was prompted by a history of maternal polyhydramnios, excessive drooling of saliva, respiratory distress and cyanotic episodes associated with feeding at birth. The diagnosis was confirmed radiologically by a plain radiograph of chest and abdomen with a red rubber catheter/an infant feeding tube in situ.
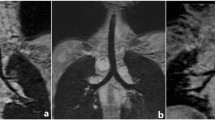
All the patients underwent standard preoperative management including clinical and radiological assessment for other associated congenital anomalies. After complete physiological stabilization, parental counseling and written informed consent, all the neonates were wrapped in warm cotton pads and transported to the CT scan suite in a properly equipped neonatal incubator. Before CT, the proximal pouch was thoroughly aspirated with a suction catheter and oxygen was provided through a nasal cannula during the whole procedure. All CT examinations were performed using a low-dose CT protocol. Contrast-enhanced spiral CT examination was performed with a HiSpeed Advantage scanner, Toshiba aquillon®, 64 slice MDCT (V3.30 ER001, Toshiba America Medical System). Nonionic contrast agent (Iohexol) was used at doses of 1.5 ml/kg. Scanning was performed at 100–120 kVp and 40–60 mA. CT dose index (CTDIvol) and CT dose length product (DLP) was noted in each case for calculation of radiation dose to the neonates. All images were obtained in the axial plane with 0.5 mm contiguous sections in a cranio-caudal direction from the larynx to the dome of the diaphragm. The scanning time was also noted. 3D CT reconstruction was performed on the Advantage Aquarius TeraRecon workstation® using 3D analysis software and multiplanar reconstruction (Fig. 1). Stored screen-saved images were obtained and after CT all the neonates underwent surgery; the CT findings were correlated with surgical results.
Type of atresia was classified as per Gross classification [5]. The 3D CT-reconstructed images were carefully evaluated for the type of esophageal atresia, the distance between the upper and lower esophageal pouches, origin, level and position of the fistula, and presence or absence of any other cardiac, pulmonary or mediastinal lesions. To avoid any motion artifacts, the first 20 neonates were sedated using midazolam intravenously in doses of 0.2 mg/kg body weight, after a due parental consent. The patients were carefully monitored during and after the examination. Sedation was avoided in the last ten cases.
Intraoperatively, the gap between the two esophageal pouches was meticulously measured following initial dissection to define the pouches, but before mobilization of pouches. A silk thread between two artery forceps was used to define the gap between the lowermost limit of the upper pouch to the highest point of the lower esophageal fistula. The silk suture was then placed alongside a standard scale and was measured in centimeters.
The patient’s individual radiation dose was obtained by calculating the effective dose, using the formula: Effective dose (E) = DLP (dose length product) × (E/DLP)age. (E/DLP)age, is an age-specific conversion factor for children undergoing chest CT examination [6]. All the further calculations were made using 0.039 as the normalized value of E/DLP [7, 8].
Statistical analysis was performed using SPSS version 17 and Graph Pad Prism 5 software. The correlation analysis was done using Pearson correlation analysis and the ‘p’ value of <0.05 was taken as significant.
Results
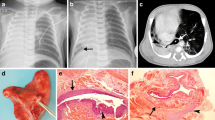
All the 30 cases had type-C EA and TEF as per Gross classification [5]. There were 22 males and 8 females. All the neonates were full term with mean birth weight of 2.41 ± 0.35 kg (range 1.8–3.37 kg). There were no procedure-related complications, but two patients developed apnea during transit from scanning suite to NSICU. In six cases, fistula could not be identified on CT scan, in the axial, sagittal or 3D reconstruction view. In another two cases, the fistula could be identified only on axial and sagittal view, but not on 3D reconstruction view. In another two cases, the lower pouch could not be seen, but the fistula could be identified as a small dimple around the carina, which was confirmed at surgery (Fig. 2).
Out of the 30 patients, end to end anastomosis was done in 26 cases (86 %) and the remaining 4 (6 %) cases underwent fistula ligation along with cervical esophagostomy and gastrostomy as primary procedure because of friable lower pouch in 3 cases and due to long gap in 1 case. Of the 26 cases with primary repair, 3 (11 %) had anastomotic leak, for which gastrostomy was done in 1 case and gastrostomy with feeding jejunostomy in the other 2 cases. Altogether, three cases had associated anorectal malformations for which additional procedures were performed in the form of anoplasty in one and colostomy in the other two cases.
The gap was classified as short, intermediate and long gap according to the classification by Brown and Tam et al. [9]. Thirteen patients had short gap (≤1 cm), 16 intermediate gap (>1 to ≤3 cm) and only 1 patient had long gap (>3 cm).
On CT scan, the mean gap between the upper pouch and lower fistula was 0.95 ± 0.57 cm (range 0.2–2.8 cm) and the mean gap at surgery was 1.38 ± 0.61 cm (range 0.5–3.2 cm). On Pearson correlation statistical analysis between the CT gap and the surgery gap, the correlation was found to be significant (p < 0.0001) and the correlation coefficient was 0.8034.
The exact location of fistula could be detected on CT scan in 24 cases. Interestingly in one case, fistula was located 1.2 cm above the carina which was confirmed at surgery, but the majority had fistula around 0.5 cm from the carina. In two cases a long upper pouch was detected on CT scan, of which one case could be identified on CT scan preoperatively, and in the other, the fistula could not be visualized at surgery. However intraoperatively, it was found to be a crossing pouch. Some additional lung and cardiac pathology could be detected on preoperative CT of the chest (Table 1). The patient’s individual radiation doses were calculated by calculating the effective dose (Table 2).
Discussion
EA with TEF is one of the most challenging congenital anomalies that are encountered by pediatric surgeons. The surgical approach depends on a correct evaluation of the tracheobronchial tree and the distance between the proximal pouch and distal fistula. Until recently, there was no proper preoperative imaging technique to evaluate the gap between the upper pouch and the distal fistula including the exact location of TEF. The interpouch gap distance has been recently regarded as a very important clinical parameter as it is related to anastomotic tension, contributing to anastomotic leakage, stricture and gastro-esophageal reflux [9, 10].
The interpouch distance can be roughly assessed by chest radiograph. When tracheal bifurcation is seen on chest radiograph, the distance between the carina and the upper pouch will give a rough idea of the interpouch length, as TEF is classically seen about 1 cm around the carina in the majority of cases [11]. However, many times it may underestimate the gap [26]. Contrast study, preoperative endoscopy and bronchoscopy do not help in accurately assessing the actual gap length, though these may help in identifying the fistula and give an idea of short or long gap [12–17]. Few other invasive methods have been reported for measuring the gap, but it needs a gastrostomy which is undesirable when primary anastomosis is the goal [18].
There are several articles supporting the usefulness of preoperative CT scan in EA with TEF cases [19–23]. These studies found CT scan to be accurate in providing information on the level of fistula and the interpouch distance. The spatial relationship between the two pouches and location of TEF help in proper orientation of the anomaly by the surgeon preoperatively. In addition, other tracheobronchial anomalies and mediastinal anomalies could be detected if present.
In the present study, all 30 cases underwent contrast-enhanced spiral CT scan preoperatively as per the protocol. The procedure was quick and non-invasive. There were no procedure-related complications, except for two neonates who developed apnea on transit and were subsequently resuscitated with a favorable outcome.
All the patients in the study group had type-C EA according to the Gross classification, which is the most common variety with incidence of 85.8 % [1, 24]. None of the patients had an associated upper pouch fistula (type D). Contrary to previous studies where TEF could be accurately detected in all cases, in the present study the distal fistula identified at surgery was neither detected in the sagittal or axial sections nor in the 3D reconstruction view in six cases (20 %). Of these, four cases had narrow fistulae and two had wide fistulae. In two cases, the fistula could not be visualized on 3D reconstruction view, but could only be appreciated as a dimple on sagittal or axial sections. These findings were further confirmed at surgery (i.e., location of dimple matched the level of TEF). The gap between the upper pouch and distal TEF could be measured in 24 cases, which on statistical correlation with the intraoperative gap measurement was found to be highly significant (p < 0.0001).
Another advantage of CT scan is detection of concomitant pathologies. Fourteen patients had lung pathology in the form of collapse or consolidation and one case had enlarged right pulmonary artery. Cardiac pathology were detected in four cases which included Fallot’s tetralogy, patent ductus arteriosus, mesocardia with double SVC draining into the right atrium and large aberrant right subclavian artery. One patient had butterfly vertebrae at the T9 level.
Radiation risk estimation
The Biological Effects of Radiation VII report 2005 estimates approximately one radiation-induced cancer per 10,000 individuals with an age distribution similar to that of the entire US population exposed to 1 mSv, of which half would be fatal [25]. It is noted that children have a nominal radiation risk that is about a factor of three higher than a population average [26]. From this information, the nominal radiation cancer risk in the present study population with mean effective dose of 1.79 mSv will be approximately 1.79 radiation-induced cancer per 10,000 individuals, half of which will be expected to be fatal. These are of the same magnitude as effective from average natural background doses (including radon exposure) each year (i.e., approximately 3 mSv/year) [27]. So, when compared to the natural background, these patients have the same magnitude of cancer risk, but in addition to the natural background radiation, these children have almost double the magnitude of cancer risk.
In the advent of new fast magnetic resonance imaging (MRI) techniques with better imaging quality and ultrafast imaging, the radiation issues will be resolved [28] and preoperative imaging with fast MRI may find its place in evaluation of EA and TEF in neonates in future.
In summary, preoperative CECT chest with 3D reconstruction in EA with TEF cases offers certain advantages:
-
It is a quick, noninvasive procedure
-
Sedation may not be required in all cases.
-
The exact site of TEF could be identified in 80 % cases accurately, thereby assessing the interpouch gap preoperatively.
-
Associated concomitant lung pathology and various cardiac pathologies can also be identified fairly.
However, there are also certain disadvantages of CT:
-
In 20 % cases, the fistula could not be identified with preoperative CT scan, but air was present in the abdomen on plain radiograph, indicating the presence of TEF which was later confirmed at surgery.
-
The effective radiation dose calculated was 1.79 mSv (mean). With this radiation dose, there would be approximately 1.79 radiation-induced cancer per 10,000 individual predicted, half of which would be expected to be fatal.
-
In addition to the low level radiation from the natural background, the additional radiation from the CT scan will definitely increase morbidity and cancer risk in future.
Some points of concern that have to be emphasized here are:
-
All the patients in the present study had Gross type-C EA, which is the most common variety (85.8 %).
-
No patient had an additional upper pouch fistula (Gross type D). As such, the incidence of upper pouch fistula is low (1.4 %) as per literature.
-
Most of the patients had short to intermediate gap interpouch gap; hence, primary end to end anastomosis was possible in most cases.
-
Preoperative CT did not change the management plan in all the cases in the present study.
-
Apart from lung consolidation, no other major pulmonary or mediastinal anomalies were detected, which are as such rare associations.
-
Cardiac evaluation is more accurate with echocardiography, which is a bedside procedure and does not require sedation.
-
There may be risk of apnea, aspiration and hypothermia during shifting the patient to or from the CT suite.
-
Children have a nominal radiation risk that is about a factor three higher than a population average.
Conclusion
Though preoperative CT scan of chest with 3D CT reconstruction has many advantages than conventional diagnostic procedures, it involves a significant exposure to ionizing radiation and risk of radiation-induced cancer in future. Despite minimizing the settings of scanning to minimize the effective dose, there is risk of low-level ionizing radiation. The most common variety of EA and TEF is the type-C TEF, which has an incidence of 86 %, and most commonly the interpouch gap is low to intermediate (97 %), which can be anastomosed primarily. The lung consolidation and collapse can also be detected on plain chest radiograph, and routine preoperative 2D echocardiography can detect all cases of congenital cardiac anomalies. The risk of ionizing radiation and potential radiation-induced cancer risk in future outweigh the potential benefits of the procedure. Preoperative CT chest with 3D CT reconstruction may play a crucial role in evaluation of the H-type fistula (Gross type E), recurrent TEF or in cases associated with complex airway pathology, but the incidence of such entities are very low.
Hence, currently there is no role of CT scan in the routine preoperative assessment of the EA with distal TEF. However, with the advent of fast MRI, it may be a good adjunct for better evaluation of these anomalies in future.
References
Harmon CM, Coran AG (2006) Congenital anomalies of esophagus. In: Grosfeld JL, O’Neill JA, Fronkalsrud EW, Coran AG (eds) Pediatric surgery. Mosby-Elsevier, Philadelphia, pp 1051–1082
Bax KN, Roskott AMC, Vander Zee DC (2008) Esophageal atresia without distal tracheoesophageal fistula: high incidence of proximal fistula. J Pedaitr Surg 43:522–525
Guo W, Li Y, Jiao A, Peng Y, Hou D, Chen Y (2010) Tracheoesophageal fistula after primary repair of type C esophageal atresia in the neonatal period: recurrent or missed second congenital fistula. J Pediatr Surg 45:2351–2355
Islam S, Cavanaugh E, Honeke R, Hirschl RB (2004) Diagnosis of proximal tracheoesophageal fistula using three-dimensional CT scan: a case report. J Pediatr Surg 39(1):100–102
Gross RE (1953) The surgery of infancy and childhood. WB Saunders, Philadelphia
Huda W (2007) Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc 4:316–320
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
Chapple CL, Willis S, Frame J (2002) Effective dose in pediatric computed tomography. Phys Med Biol 47:107–115
Brown AK, Tam PK (1996) Measurement of gap length in esophageal atresia: a simple predictor of outcome. J Am Coll Surg 182:41–45
Upadhyaya VD, Gangopadhyaya AN, Gupta DK, Sharma SP, Kumar V et al (2007) Prognosis of congenital tracheoesophageal fistula with esophageal atresia on the basis of gap length. Pediatr Surg Int 23:767–771
Van der Zee DC, Vieirra-Travassos D, De Jong JR, Tytgat SH (2008) A novel technique for risk calculation of anastomotic leakage after thoracoscopic repair for esophageal atresia with distal fistula. World J Surg 32:1396–1399
Rossi C, Domini M, Aquino A, Pesico A, Lelli-Chiesa P (1998) A simple and safe method to visualize the inferior pouch in esophageal atresia without fistula. Pediatr Surg Int 13:535–536
Koplewitz BZ, Udassin R (2003) Radiographic assessment of the gap between oesophageal pouches in infants with oesophageal atresia without fistula. Eur J Pediatr 162:650–651
Atzori P, Iacobelli BD, Bottero S et al (2006) Preoperative tracheobronchoscopy in new borns with esophageal atresia: does it matter? J Pediatr Surg 41(6):1054–1056
de Blic J, Marchac V, Scheinmann P (2002) Complications of flexible bronchoscopy in children: prospective study in 1,328 procedures. Eur Respir J 20:1271–1276
Ianolli ED, Litman RS (2002) Tension pneumothorax during flexible fiberoptic bronchoscopy in a newborn. Anesth Analg 94:512–513
Garcia NM, Thompson JW, Shaul DB (1998) Definitive localization of isolated tracheoesophageal fistula using bronchoscopy and esophagoscopy for guide wire placement. J Pediatr Surg 33:1645–1647
Chang KL, Saing H (1995) Combined flexible endoscopy and fluoroscopy in assessment of gap between the two esophageal pouches in esophageal atresia without fistula. J Pediatr Surg 30(5):668–670
Johnson JF, Sueoka BL, Mulligan ME, Lugo EJ (1985) Tracheoesophageal fistula: diagnosis with CT. Pediatr Radiol 15:134–135
Tam PKH, Chan FL, Saing H (1987) Diagnosis and evaluation of esophageal atresia by direct sagittal CT. Pediatr Radiol 17:68–70
Ratan SK, Varshney A, Sumita M, Saxena NC, Kakar S, Sodhi PK (2004) Evaluation of neonates with esophageal atresia using chest CT scan. Pediatr Surg Int 20:757–761
Lam WWK, Tam PKH, Chan FL, Chan KL, Cheng W (2000) Esophageal atresia and tracheal stenosis: use of three-dimensional CT and virtual bronchoscopy in neonate, infants and children. AJR Am J Roentgenol 174:1009–1012
Fitoz S, Atasoy C, Yagmurlu A, Akyar S, Erden A, Dindar H (2000) Three-dimensional CT of congenital esophageal atresia and distal tracheoesophageal fistula in neonates: preliminary results. AJR Am J Roentgenol 175:1403–1407
Beasley S (2009) Congenital malformation. In: Parikh DH, Crabbe DCG, Auldist AW, Rothenberg SS (eds) Pediatric thoracic surgery. Springer, London, pp 281–309
National Academy of Sciences Committee on the Biological Effects of Ionizing Radiation (2005) Report VII (BEIR VII, Phase 2): health risks from exposure to low levels of ionizing radiation. National Research Council, Washington. http://books.nap.edu/catalog/11340.html
Brenner DJ, Elliston CD, Hall EJ et al (2001) Estimated risks of radiation induced fatal cancer from pediatric CT. Am J Roentgenol 176:289–296
United Nations Scientific Committee on the Effects of Atomic radiation (UNSCEAR) (2000) Sources and effects of ionizing radiation. United Nations, New York
Piccolo RL, Bongini U, Basile M, Savelli S et al (2012) Chest fast MRI: an imaging alternative on preoperative evaluation of pectus excavatum. J Pediatr Surg 47:485–489
Acknowledgments
We would like to thank Dr. Dhananjay Vaze, senior registrar, Pediatric Surgery, PGIMER, Chandigarh, for his invaluable effort for helping in editing the manuscript and few vital suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahalik, S.K., Sodhi, K.S., Narasimhan, K.L. et al. Role of preoperative 3D CT reconstruction for evaluation of patients with esophageal atresia and tracheoesophageal fistula. Pediatr Surg Int 28, 961–966 (2012). https://doi.org/10.1007/s00383-012-3111-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3111-9