Abstract
Aim
The purpose of this study was to compare the outcome of infants having antenatally detected urinary tract abnormalities (AUTAs) with respect to the presence of hydronephrosis in postnatal ultrasonography (US) examination.
Patients and methods
Between January 1999 and October 2009, 256 infants diagnosed with AUTAs were prospectively followed. Infants were divided into two groups according to the presence of hydronephrosis in postnatal US examination: Group 1, infants with hydronephrosis; Group 2, infants without hydronephrosis (including renal cyst, agenesis, ectopic kidney). The events of interest were the presence and diagnoses of uropathy, AUTA resolution, urinary tract infection (UTI), development of renal parenchymal defects (RPDs)—focal or global scarring, dysplasia—, acute kidney injury (AKI) and chronic kidney disease (CKD), and the need for surgery and dialysis treatment.
Results
The most commonly detected underlying abnormalities were ureteropelvic junction obstruction (44.8 %), vesicoureteral reflux (VUR) (30.0 %) and megaureter (9.5 %) in patients with postnatal hydronephrosis. On the other hand, multicystic dysplastic kidney (43.5 %), renal agenesis (19.4 %) and VUR (19.4 %) were mostly encountered abnormalities in patients without postnatal hydronephrosis. RPDs were significantly more common among patients with postnatal hydronephrosis compared to those without hydronephrosis (37 vs. 21 %, P = 0.02). The incidence of UTI and VUR was higher in infants with postnatal hydronephrosis than in infants without hydronephrosis. There was no statistically significant difference in terms of the development of AKI and CKD and the need for surgery and dialysis treatment between patients with hydronephrosis and those without hydronephrosis.
Conclusion
Infants with AUTAs should be investigated postnatally. The findings from this study will help to identify the natural history and outcome of infants with AUTAs according to the postnatal US parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antenatally detected urinary tract abnormalities (AUTAs) are the commonest anomalies detected on prenatal ultrasonography (US), accounting for 20–50 % of all congenital disorders and about 50 % of these abnormalities manifest as hydronephrosis [1, 2]. The most common anomalies associated with AUTAs are vesicoureteral reflux (VUR), obstructive uropathies, multicystic dysplastic kidney (MCDK) and congenital megaureter [3–5]. If these anomalies are not detected by the antenatal US, they may manifest later in life as pyelonephritis (PN), hypertension (HT), complications during pregnancy or even end-stage renal disease (ESRD). The actual etiology of renal parenchymal defects (RPDs) remains controversial. It is important to recognize that pyelonephritis can occur in congenitally abnormal kidneys, so that injury in an individual patient might be both congenital and acquired. Acquired renal scarring is different from a diffuse congenital renal scarring (hypodysplasia). In contrast to acquired renal scarring, congenital renal scarring cannot be prevented. Therefore, early diagnosis is crucial in some cases, as potential targeted therapy might prevent irreversible damage of the renal parenchyma. In adequately treated infants, renal damage is probably due to preexisting, congenital renal parenchymal pathology and not to the inflammatory process.
In most cases, US is able to differentiate between a normal and abnormal fetal urinary tract [3]. But attempts to use antenatal US data to predict postnatal outcome has not been successful to date because even a minor AUTAs can coexist with various types of anomalies, especially VUR, with known association to nephro-urological morbidity [4, 5]. By shifting attention from antenatal to postnatal US analysis, more recent studies have also shown that infants with normal kidneys and urinary tract and infants with significant urinary tract abnormalities can be detected [5–9]. To date, correlation between the postnatal US findings and clinical outcome in children with AUTAs has not been established. The primary objective of this study was to compare the diagnoses, natural history, renal function, and the need for postnatal treatment of children with AUTAs with respect to the presence of hydronephrosis in postnatal US examination. The secondary objectives were to assess the consistency of prenatal and postnatal US findings and define a follow-up protocol for infants with two normal postnatal US examinations.
Patients and methods
Patients
In this study, 256 infants referred to our Nephrology Unit who were found to have AUTAs between January 1999 and October 2009 underwent systematic investigation for urinary tract abnormalities and were prospectively followed. The prenatal US examination had been performed once or several times at various stages in pregnancy for obstetric reasons at the Department of Obstetrics, Marmara University Hospital, Istanbul and other Obstetrics Centres in different parts of Istanbul. If the US examinations were performed during the 15th to 19th gestational weeks, follow-up examinations were carried out after the 20th week of gestation when anomalies had been detected or there were various obstetric indications. All infants whose antenatal sonography obtained after the 20th week of gestation that showed AUTAs (e.g., hydronephrosis, renal cyst, agenesis, hypoplasia, ectopic kidney) and referred to our Pediatric Nephrology Unit in the first week of life were included in the study. Parents were informed by being given an explanation of the clinical significance of urinary tract abnormalities and the rationale of follow-up.
Postnatal investigations
All 256 patients had two successive postnatal US examinations of the urinary tract on days 7–10 and the first month of life. Criteria for abnormal postnatal US included hydronephrosis (pelvic diameter ≥5 mm, calyceal or ureteral dilatation), signs of renal hypodysplasia (small kidney, cortical cysts), renal agenesis and abnormally located kidney (ectopic kidney). According to our previous protocol that we accepted and used in those days, voiding cystouretherography (VCUG) was performed in all infants during the fourth to eighth weeks of life irrespective of the postnatal US findings. VUR was classified according to a grading system of the international classification system, which grades VUR from I to V [10]. When initial examinations suggested the presence of a nephro-urological abnormality, follow-up was individualized, and additional relevant radiological investigations [dimercaptosuccinic acid (DMSA) and dimemercapto-acetyltriglycine (MAG-3) scintigraphy] were carried out. If the VCUG showed the presence of VUR, a DMSA scan was performed after the third month of life. Even if VUR was not present, a DMSA scan was routinly undertaken for those children whose postnatal US examination showed abnormality other than hydronephrosis (renal cysts, agenesis or ectopia). RPDs were defined as focal or multifocal perfusion defects detected at the first DMSA examination persisted on the follow-up of DMSA examination or as a split renal uptake of <45 % [5]. Detection of hydronephrosis on postnatal US in association with normal VCUG led us to perform MAG-3 scintigraphy. Obstructive uropathy was diagnosed if the patient had partial response or no response to furosemide administration on MAG-3. Other investigations, such as intravenous pyelography, magnetic resonance urography or computed tomography were only undertaken after discussion at weekly nephrouroradiology meetings.
Antibiotic prophylaxis policy
Antibiotic prophylaxis with amoxicillin 10 mg/kg/day, a single night dose was started at birth for all infants with antenatal hydronephrosis (AH) and replaced by trimethoprim 1–2 mg/kg/day, a single night dose when they were 6 months old if needed. On admission, urinalysis and urine culture tests were performed. Urinary tract infection (UTI) was defined as growth of at least 105 CFU/mL bacteria in bagged specimens. Urine specimens for culture were carefully collected at our hospital by trained technicians. For infants, to avoid fecal contamination, the urine specimen was collected with a sterile bag after complete cleaning of the entire perineal area. Analysis of a bag-collected specimen as a non-invasive diagnostic procedure was a reasonable screening test in those infants, as long as they did not appear so ill as to warrant the initiation of antimicrobial therapy. Those who thought to be given antimicrobials on clinical grounds had a second specimen obtained for culture by transuretheral catheterization that is unlikely to be contaminated. Infants with proven UTI were treated with suitable antibiotics. Antibiotic prophylaxis was discontinued at 3 months for patients, if VCUG was normal and no further UTIs occured. On the other hand, antibacterial prophylaxis was continued until the resolution of reflux for patients with VUR. For infants with AUTAs other than hydronephrosis, antibiotic prophylaxis was not routinely started at birth. In those infants, if the VCUG showed the presence of VUR, antibacterial prophylaxis was started. We tried to improve medication compliance by enhancing patient/parent–doctor communication and used indirect methods mostly, patient/caregiver questionnaire, to measure compliance.
Definitions
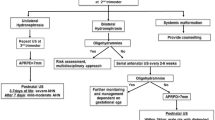
Infants were divided into two groups according to the presence of hydronephrosis in postnatal US examination: Group 1, infants with hydronephrosis; Group 2, infants without hydronephrosis (including renal cyst, agenesis, ectopic kidney) (Fig. 1). The diagnosis considered to be the single most significant which was accepted as principal diagnosis or underlying abnormality; for example, if one patient with MCDK on the right side also had contralateral VUR and duplex ureter at further work-up (antenatal and postnatal US examinations showed renal cysts on the right kidney with a normal left kidney), MCDK was accepted as principal diagnosis while VUR and duplex ureter were accepted as accompanying urinary tract abnormalities. In the absence of postnatal recognized uropathy, AUTAs were regarded as a non-significant abnormality (NSA) in the analysis. Those renal units with fetal renal pelvis dilatation not confirmed on postnatal US or returned to normal on repeated US examinations and negative findings on VCUG were labeled as transient dilatation (TD) and were also included in the group of NSAs.
Statistical analysis
Data were described as frequencies and means with standard deviations (SD) unless otherwise indicated. The normality Kolmogorov–Smirnov test was performed to determine whether or not continuous variables were normally distributed. Differences between groups were assessed by Chi-squared analysis for categorical variables. An interrater reliability analysis using the kappa statistic was performed to determine consistency among pre- and postnatal ultrasound examinations for each renal abnormality. By using renal scarring as a dependent variable in multiple logistic regression model, independent predictors were determined. Variables associated univariately were included in the multiple regression model (forward stepwise selection). All analysis were performed using SPSS Version 11.5 statistical software using default settings, P < 0.05 was set as the level of statistical significance.
Results
Population characteristics
Over the 10-year period, 256 infants [512 kidney units (KUs)] were referred with AUTAs. The demographic data are shown in Table 1. Antenatal US examination showed abnormalities in 345/512 KUs. The most common renal abnormality in antenatal US examination was hydronephrosis (78.9 %), followed by renal cysts (10.5 %) (Table 2). Other abnormalities included renal agenesis (3.9 %), renal hypoplasia (1.2 %) and ectopic kidney (1.2 %). Overall, antenatal renal abnormalities appeared to be primarily a problem of boys (187 males, 69 females; with a M:F ratio of 2.71), largely due to the remarkable predominance of males (77.7 % males vs. 22.3 % females) among patients with hydronephrosis. Meanwhile, renal cysts affected both sexes equally (48.1 % females vs. 51.9 % males). Although left kidney was affected by hydronephrosis more than right kidney, both sides were almost equally affected by other pathologies (Table 2).
Consistency of pre- and postnatal US findings
The interrater reliability for the pre- and postnatal US examinations for the identification of each renal abnormality were as follows: for detecting hydronephrosis, κ = 0.67 (P < 0.0001); for renal cysts, κ = 0.572 (P < 0.0001); for renal hypoplasia, κ = −0.006 (P = 0.9). Of the 345 KUs, 311 units were found to be abnormal in postnatal US examination (Table 2). Out of 202 patients who had been reported to have hydronephrosis in antenatal US examination 183 were confirmed to have hydronephrosis by postnatal US (Fig. 1). On the other hand, out of 54 patients reported to have no hydronephrosis in antenatal USG, 11 were found to have hydronephrosis in the postnatal US examination. Overall, among the 256 infants who had been referred due to suspected renal abnormality in antenatal US, only 13 had two normal postnatal US examinations. In the follow-up of those 13 patients, 5 patients developed no renal disease or UTI and diagnosed as NSA. On the other hand, five of those had VUR, of which three were bilateral; four patients with VUR had also RPDs and three of them were operated. All five infants with VUR developed recurrent UTI while on prophylactic treatment. The remaining three patients have yet to complete diagnostic evaluation.
Outcome of renal abnormalities with respect to postnatal ultrasonographic findings
The complete diagnostic protocol was completed in 86 % (220/256) of all included infants. Of the 220 patients, 162 (210 KUs) had hydronephrosis in postnatal US examination (Fig. 1). Diagnostic work-up of patients with hydronephrosis in postnatal examination revealed ureteropelvic junction obstruction (UPJO) in 94 KUs (44.8 %), VUR in 63 (30.0 %), megaureter in 20 (9.5 %), posterior urethral valve (PUV) in 16 (7.6 %), ureterovesical junction obstruction (UVJO) in 6, duplex system in 5, extrarenal pelvis in 3, mid-ureteric stricture in 1 and MCDK in 2 (Table 3). Of the 220 patients, 58 (62 KUs) had findings other than hydronephrosis (renal cysts, agenesis, ectopia or normal) in postnatal US examination (Fig. 1). Underlying abnormalities were MCDK in 27 KUs (43.5 %), renal agenesis in 12 (19.4 %), VUR in 12 (19.4 %), ectopic kidney in 5 (8.1 %), UPJO in 3 and crossed-fused ectopia, renal hypoplasia, ureterocele in 1 (Table 3). In this cohort, 10 infants (6 %) were found to have TD and 12 infants (5.5 %) were diagnosed as NSA (Fig. 1). Among those 12 patients, 7 had hydronephrosis in postnatal US examination while 5 had normal findings. None of the patients with abnormality other than hydronephrosis at postnatal US scan was diagnosed as NSA.
Both principle diagnoses and accompanying urinary tract abnormalities of 220 patients (440 KUs) are shown in Table 4. Of these 440 postnatally observed renal units, 333 urinary tract anomalies were detected. The following were among the most commonly encountered abnormalities: UPJO in 100 KUs (30 %), VUR in 94 (28 %), MCDK in 29 (9 %), megaureter in 23 (7 %), duplex ureter in 18 (5 %), renal agenesis in 13 (4 %) and ureterocele in 11 (3 %). The most frequently seen accompanying urinary tract abnormality was VUR; additional 19 refluxing units were detected. Associated urological anomalies were noted in eight (30 %) patients with MCDK. This included contralateral VUR in 5 (19 %), ipsilateral VUR in 1, contralateral UPJO in 1 and bilateral ureterocele in 1. Patient with UPJO required pyeloplasty, whereas four patients with VUR and patient with bilateral ureterocele were managed conservatively.
During the follow-up, 37 % of patients had UTIs and 33 % had RPDs. RPDs were significantly related to UTI (RPDs were detected in 52.5 % of the patients with UTIs vs. 14.9 % of the patients without UTIs, P < 0.0001). While UTIs were not specifically related to hydronephrosis or renal cysts, RPDs were significantly more common among patients with hydronephrosis compared to those without hydronephrosis (37 vs. 21 %, P = 0.02, Table 5). In the logistic regression analysis, UTI, hydronephrosis and VUR were found as independent risk factors for RPD formation (Table 6).
Seventy-seven of 256 children (30 %) required surgery for various indications (Table 7). The largest group consisted of patients with VUR who required ureteric reimplantation. Sixty-one patients with hydronephrosis (31 %) and 16 without hydronephrosis (26 %) at postnatal US required surgery (P > 0.05). The type of surgery with respect to VUR, UPJO, PUV, ureterocele and megaureter are shown in Table 7.
During the follow-up, seven patients developed acute kidney injury (AKI) and ten developed chronic kidney disease (CKD), where two of AKIs and two of CKDs required dialysis. No statistically significant difference in terms of the development of AKI and CKD and need for surgery and dialysis treatment was found between patients with hydronephrosis and without hydronephrosis (Table 5). Three patients died: one with bilateral hydronephrosis (diagnosed as bilateral MCDK at autopsy), one with bilateral renal cysts (diagnosed as bilateral MCDK at autopsy) and one with right renal agenesis and left hypodysplastic kidney (reported as bilateral agenesis in antenatal US).
Discussion
The prediction of renal abnormalities from antenatal US examination has not been successful to date. This poor correlation between antenatal findings and postnatal diagnosis may be due to technical difficulties with imaging of the renal tract, TD and other possible anatomical changes during gestation. In this study, we addressed the use of postnatal US instead of antenatal US to predict the presence of urinary tract abnormalities because complete details of the antenatal studies were often unavailable. Moreover, since all children with AUTAs underwent postnatal US, it is usually the latter that determines the need for further investigations. In the present study, antenatal US findings changed in almost one-sixth (16 %) of the cases at postnatal US examination. As previously reported by Najmaldin et al. [11], errors included inability to distinguish between mostly cystic dysplasia and hydronephrosis, and hydronephrosis and normal patients (Fig. 1).
In the normal neonate, there is low urine output during the first 24–48 h so that with unilateral hydronephrosis the US should not be undertaken before 72 h post-delivery [12]. Radiologists advise delaying US until urine output has recovered postnatally. This is usually after day 5. Thus, US was performed routinely on days 7–10 and at the first month of this study. The normal result of the first US was not considered in exclusion of the second one.
One of the issue under debate is whether continued observation is necessary when both postnatal US results are normal. VCUG carries risks involving significant radiation exposure and risk of UTI. Ismaili et al. [13] demonstrated that when 2 neonatal US scans were normal, the VCUG showed an abnormality in only 5/74 (6.7 %) of the infants. Avni et al. [6] showed that a careful and meticulous US examination of the neonatal urinary tract allows the detection of over 87 % of refluxing renal units by showing at least one sonographic abnormality. Lidefelt and Herthelius performed two US examinations on the fifth to seventh day and during the third week of life in 103 infants with AH, all of whom underwent VCUG. When both neonatal US findings were normal, the VCUG showed an abnormality in only 3/53 (5.6 %) of the infants [7]. VCUG showed reflux grade 1 in all of these three infants. Because of these concerns, many authors have used results from postnatal US to select children at greater risk for VUR and consequently who should undergo VCUG [14]. However, our investigation protocol differed significantly in that we performed VCUG on all babies with AUTA even if both postnatal US scans were normal. Thus, a diagnosis of VUR was made in five babies who had two normal postnatal US scans. The importance of looking for reflux, even in the presence of a normal postnatal US scan, is supported by the findings of Jaswon et al. [15], Tibballs and De Bruyn [16], Phan et al. [17] and Aksu et al. [2]. Aksu et al. [2] reported that 23 (45 %) of 51 patients referred for treatment of AH whose first postnatal US was normal were diagnosed postnatally as having urinary tract abnormality. These findings suggest that normal postnatal US examination does not exclude VUR and patients should continue to be monitored.
In this study, when both postnatal US results were normal, the VCUG showed VUR in 5/10 (50 %) of the infants. All five infants with VUR had recurrent UTIs while on prophylactic treatment. Remaining five infants were diagnosed as NSA and none of them developed UTI. As a result, our findings suggest that urinary tract abnormalities can sometimes be overlooked by US. In these cases, we suggest that recurrent UTIs should be a key indicator for reflux, and all infants with AUTA who had normal postnatal US examination and recurrent UTIs should be subjected to VCUG. On the other hand, our findings suggest that VCUG is not advisable in an asymptomatic infant with a normal postnatal US.
The results of our study showed that the rate of TD was 6 % and the rate of NSA was 5.5 %. Postnatal US examination of patients with NSA revealed either hydronephrosis or normal result. No patient with NSA had abnormality other than hydronephrosis at postnatal US scan which means that there was not any such resolution in infants without hydronephrosis. Mild prenatal hydronephrosis may be an expression of the physiological changes associated with normal growth and development of the fetal renal pelvis, coupled with increased fetal urinary output [18]. Although most other studies have demonstrated higher total resolution rates [19, 20], our results are consistent with a recent report demonstrating spontaneous resolution in 13 % of patients [2]. Our study demonstrated lower resolution rate, perhaps because of preselected study populations weighted by more severe cases, the different diagnostic criteria used, or of variations in diagnostic imaging modalities utilized in the postnatal period. The results of our study showed that the rate of spontaneous resolution even in infants with normal postnatal US was 50 % (5 of 10 infants) and the rate of surgery was 30 %. Given the results from our study, we suggest that any infant with AUTAs should not be considered clinically insignificant and be followed.
In this study, underlying diseases are shown in Table 3. UPJO and VUR were the most common anomalies in patients with hydronephrosis at postnatal US scan, as reported in the literature, whereas MCDK, renal agenesis and VUR were the most common anomalies in patients without hydronephrosis. Our study also confirms that postnatal hydronephrosis was more common in boys compared to girls (Table 5), but they were not at higher risk for VUR or other anomalies compared with girls with postnatal pelviectasias as reported by Phan et al. [17]. These findings suggest that the management of postnatal pelviectasias in boys and girls does not need to be sex specific.
The aim of the detection of fetal uropathies is to prevent renal damage. The onset of RPDs usually occurs early in life, usually before 5 years of age and most frequently before 3 years of age. In the postnatal period, infants with reflux previously detected to have a prenatal pelvic dilatation usually show renal damage, possibly congenital, at the time of diagnosis. Similarly, most infants in our study had early signs of RPD (scars, hypoplasia/dysplasia) on DMSA scan, probably more of congenital than of postinfectious origin. This hypothesis was supported by the finding that there was no difference in terms of the development of AKI and CKD between patients with hydronephrosis and those without hydronephrosis. However, renal damage is claimed to progress after UTIs [21]. Recurrent UTIs, pressure caused by reflux of sterile urine and intrarenal reflux are the recognized causes of RPD in infants with VUR [22]. In our study, we demonstrated that RPDs were significantly more common among patients with hydronephrosis compared to those without hydronephrosis, and UTI, hydronephrosis and VUR were detected to be risk factors for RPD formation. We think that the higher incidence of VUR and UTI during follow-up in infants with postnatal hydronephrosis than in infants without hydronephrosis explains the association between RPDs and postnatal hydronephrosis.
There are some limitations in our study, in particular, the use of retrospective data from prenatal examination, which was not standardized. So, we did not perform prenatal grading. In addition, although there is a lack of consensus, we routinely performed VCUG in all infants with AUTAs and DMSA scan in all infants with all-grade VUR. On the other hand, the advantages of our study are that our sample size was larger than that in most of the previous publications providing greater weight to the results obtained, and we had a long follow-up period. And probably most importantly, to the best of our knowledge, this is the first study comparing the natural history and outcome of infants having AUTAs with respect to the presence of hydronephrosis in postnatal US examination.
In conclusion, our data suggest that infants with AUTAs should be investigated postnatally. RPDs were significantly more common among patients with postnatal hydronephrosis compared to those without hydronephrosis. There was no statistically significant difference in terms of the development of AKI and CKD and the need for surgery and dialysis treatment between patients with hydronephrosis and those without hydronephrosis. There was poor correlation between prenatal and postnatal US findings; thus, we suggest that management decisions such as whether or not to obtain additional radiological evaluation should be based upon the findings of postnatal US. Our findings also suggest that normal postnatal US results do not exclude VUR and all infants with AUTA and recurrent UTIs should be subjected to VCUG. Further prospective, multicenter, controlled studies are needed to clarify these issues.
References
Helin I, Persson PH (1986) Prenatal diagnosis of urinary tract abnormalities by ultrasound. Pediatrics 78:879–883
Aksu N, Yavaşcan O, Kangin M, Kara OD, Aydin Y, Erdoğan H, Tuncel TC, Cetinkaya E, Ozbay E, Sandikçioğlu TG (2005) Postnatal management of infants with antenatally detected hydronephrosis. Pediatr Nephrol 20:1253–1259
Toiviainen-Salo S, Garel L, Grignon A, Dubois J, Rypens F, Boisvert J, Perreault G, Decarie JC, Filiatrault D, Lapierre C, Miron MC, Bechard N (2004) Fetal hydronephrosis: is there hope for consensus? Pediatr Radiol 34:519–529
Persutte WH, Koyle M, Lenke RR, Klas J, Ryan C, Hobbins JC (1997) Mild pyelectasis ascertained with prenatal ultrasonography is pediatrically significant. Ultrasound Obstet Gynecol 10:12–18
Lidefelt KJ, Herthelius M, Soeria-Atmadja S (2009) Antenatal renal pelvis dilatation: 2-year follow-up with DMSA scintigraphy. Pediatr Nephrol 24:533–536
Avni EF, Ayadi K, Rypens F, Hall M, Schulman CC (1997) Can careful ultrasound examination of the urinary tract exclude vesicoureteric reflux in the neonate? Br J Radiol 70:977–982
Lidefelt KJ, Herthelius M (2008) Antenatal hydronephrosis: infants with minor postnatal dilatation do not need prophylaxis. Pediatr Nephrol 23:2021–2024
Hálek J, Flögelová H, Michálková K, Smakal O, Dubrava L, Zapletalová J, Janout V (2010) Diagnostic accuracy of postnatal ultrasound screening for urinary tract abnormalities. Pediatr Nephrol 25:281–287
Grazioli S, Parvex P, Merlini L, Combescure C, Girardin E (2010) Antenatal and postnatal ultrasound in the evaluation of the risk of vesicoureteral reflux. Pediatr Nephrol 25:1687–1692
Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Mobius TE (1985) International system of radiographic grading of vesicoureteral reflux. International Reflux Study of Children. Pediatr Radiol 15:105–109
Najmaldin AS, Burge DM, Atwell JD (1990) Antenatally diagnose urological abnormalities: where do we stand? Pediatr Surg Int 5:195–197
de Bruyn R, Gordon I (2001) Postnatal investigation of fetal renal disease. Prenat Diagn 21:984–991
Ismaili K, Avni FE, Hall M (2002) Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr 141:21–24
Mallik M, Watson AR (2008) Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol 23:897–904
Jaswon MS, Dibble L, Puri S, Davis J, Young J, Dave R, Morgan H (1999) Prospective study of outcome in antenatally diagnosed renal pelvis dilatation. Arch Dis Child Fetal Neonatal Ed 80:F135–F138
Tibballs JM, De Bruyn R (1996) Primary vesicoureteric reflux—how useful is postnatal ultrasound? Arch Dis Child 75:444–447
Phan V, Traubici J, Hershenfield B, Stephens D, Rosenblum ND, Geary DF (2003) Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol 18:1224–1228
Woodward M, Frank D (2002) Postnatal management of antenatal hydronephrosis. BJU Int 89:149–156
Sairam S, Al-Habib A, Sasson S, Thilaganathan B (2001) Natural history of fetal hydronephrosis diagnosed on mid-trimester ultrasound. Utrasound Obstet Gynecol 17:191–196
Koff SA (2000) Postnatal management of antenatal hydronephrosis using an observational approach. Urology 55:609–611
Penido Silva JM, Oliveira EA, Diniz JS, Bouzada MC, Vergara RM, Souza BC (2006) Clinical course of prenatally detected primary vesicoureteral reflux. Pediatr Nephrol 21:86–91
Stock JA, Wilson D, Hanna MK (1998) Congenital reflux nephropathy and severe unilateral fetal reflux. J Urol 160:1017–1018
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gokce, I., Biyikli, N., Tugtepe, H. et al. Clinical spectrum of antenatally detected urinary tract abnormalities with respect to hydronephrosis at postnatal ultrasound scan. Pediatr Surg Int 28, 543–552 (2012). https://doi.org/10.1007/s00383-012-3072-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3072-z