Abstract
Purpose
The exact pathogenesis of pulmonary hypoplasia in the nitrofen-induced congenital diaphragmatic hernia (CDH) still remains unclear. Smad1, one of the bone morphogenesis protein (BMP) receptor downstream signaling proteins, plays a key role in organogenesis including lung development and maturation. Smad1 knockout mice display reduced sacculation, an important feature of pulmonary hypoplasia. Wnt inhibitor factor 1 (Wif1) is a target gene of Smad1 in the developing lung epithelial cells (LECs). Smad1 directly regulates Wif1 gene expression and blockade of Smad1 function in fetal LECs is reported to downregulate Wif1 gene expression. We designed this study to test the hypothesis that pulmonary Smad1 and Wif1 gene expression is downregulated during saccular stage of lung development in the nitrofen CDH model.
Methods
Pregnant rats were exposed to either olive oil or nitrofen on day 9 of gestation (D9). Fetuses were harvested on D18, and D21. Fetal lungs were dissected and divided into 2 groups: control and nitrofen (n = 9 at each time point, respectively). Pulmonary gene expression of Smad1 and Wif1 were analyzed by real-time RT-PCR. Immunohistochemistry was performed to evaluate protein expression/distribution of Smad1 and Wif1.
Results
The relative mRNA expression levels of Smad1 and Wif1 were significantly downregulated in the nitrofen group compared to controls on D18 and D21 (*p < 0.01, **p < 0.05). Immunoreactivity of Smad1 and Wif1 was also markedly decreased in nitrofen lungs compared to controls on D18 and D21.
Conclusion
We provide evidence, for the first time, that the pulmonary gene expression of Smad1 and Wif1 is downregulated on D18 and D21 (saccular stage of lung development) in the nitrofen-induced hypoplastic lung. These findings suggest that the downregulation of Smad1/Wif1 gene expression may contribute to pulmonary hypoplasia in the nitrofen CDH model by retardation of lung development during saccular stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) occurs in approximately 1 in 2,500 newborns [1]. Despite improved prenatal diagnostic techniques and advances in neonatal intensive care, the mortality for CDH remains high due to severe pulmonary hypoplasia (PH) and persistent pulmonary hypertension [2–4]. However, the exact mechanism of PH in CDH is still unknown.
Nitrofen-induced CDH model has been used extensively as an experimental model because of the occurrence of the diaphragmatic defect and associated pulmonary hypoplasia, in this model which is strikingly similar to the human condition. Maternal exposure to nitrofen on day 9 of gestation in rodents results in 100% lung hypoplasia and high rate (40–80%) of CDH in the offspring [5]. Although studies from our laboratory have recently shown that several signaling pathways are altered in the nitrofen-induced hypoplastic lung, the exact mechanisms by which nitrofen causes PH remains unclear [6–8].
Smad1, one of the bone morphogenetic protein (BMP) receptor downstream signaling proteins, plays a key role in organogenesis including lung development and maturation [9]. Smad1 is expressed in the developing lung epithelium as well as in the surrounding mesenchyme [10]. A previous study from our laboratory has shown that gene expression of BMP4 is downregulated in the nitrofen-induced hypoplastic lung in early gestation [11]. Recently, it has been reported that the BMP signaling system is active during late lung development [12]. It has also been reported that lungs of Smad1 knockout mice display reduced sacculation [13]. Furthermore, Wnt inhibitor factor 1 (Wif1) has recently been shown as a target gene of Smad1 in the developing lung epithelial cells (LECs) [13]. Blockade of Smad1 function in fetal lung epithelial cells results in downregulation of Wif1 gene expression [13]. We, therefore, designed this study to test the hypothesis that pulmonary Smad1 and Wif1 gene expression is downregulated during saccular stage of lung development in the nitrofen CDH model.
Materials and methods
Animals and drugs
Adult Sprague-Dawley rats were mated, and the presence of spermatozoids in the vaginal smear was considered as proof of pregnancy. The day of this observation was determined as gestational day 0 (D0). Pregnant rats were randomly divided into two groups. On D9 (term, 22 days), the rats in the experimental group received 100 mg of nitrofen (WAKO Chemicals, Osaka, Japan) dissolved in 1 ml of olive oil intragastrically under short anesthesia, whereas those in control received only vehicle. Fetuses were harvested by cesarean section on D18 and D21. Fetal lungs were divided into 2 groups: control (n = 9) and nitrofen (n = 18) on D18 and D21. In order to obtain representative numbers, the fetuses in each group came from at least four different dams. The Department of Health and Children approved the protocol of these animal experiments (ref. B100/4142) under the Cruelty to Animals Act, 1876; as amended by European Communities Regulations 2002 and 2005.
RNA extraction and real-time reverse transcription polymerase chain reaction
The peripheral region of the left lung of each fetus was suspended in TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) immediately after dissection, quickly frozen in liquid nitrogen and stored at −20°C. After thawing frozen samples, they were homogenized with a pellet pestle (Daigger, Vernon Hills, USA) and total RNA of the lung tissue was isolated according to recommended protocol (Invitrogen). Total RNA quantification was performed spectrophotometrically (ND–1000 UV-Vis® Spectrophotometer, NanoDrop, Wilmington, USA). Synthesis of cDNA was performed using Transcript High Fidelity cDNA Synthesis Kit® (Roche Diagnostics, Grenzach-Whylen, Germany) according to the manufacturer’s protocol. Following RT at 44°C for 60 min, real-time polymerase chain reaction was performed using LightCycler® 480SYBR Green I Master (Roche Diagnostics) according to the manufacturer’s protocol. The specific primer set used in this study is listed in Table 1. After initial denaturation step of 5 min at 95°C, 45 cycles of amplification for each primer was carried out. Each cycle included a denaturation steps; 10 s at 95°C, an annealing step; 15 s at 72°C. Final elongation temperature was 65°C for 1 min. Relative levels of gene expression were measured by Light Cycler® 480 (Roche Diagnostics) according to the manufacturer’s instruction. The mRNA expression levels of Smad1 and Wif1 were normalized to β-actin mRNA expression levels in each sample.
Immunohistochemistry
The paraffinized left lungs were sectioned at a thickness of 5 μm, and the sections were deparaffined with xylene and then rehydrated through ethanol and distilled water. Tissue sections were immersed in target retrieval solution (DAKO, Cambridgeshire, UK) in a microwave oven (at 750 W) for 15 min followed by 10 min incubation in 0.3% H2O2 to block endogenous peroxidase activity. Sections were incubated at 4°C overnight with each of the primary antibodies including a 1:100 dilution of rabbit polyclonal antibodies specific for Smad1 (LOT ab-63356; 1:500, Abcam plc, Cambridge, UK) and Wif1 (LOT sc-59949; 1:50, Santa Cruz Biotechnology, Santa Cruz, USA) and treated in horseradish peroxidase (HRP)-labeled anti-rabbit secondary antibodies (Lot: K4003; DAKO Ltd, Cambridgeshire, UK). Sections were then developed with a diaminobenzidine (DAB)-H2O2 substrate complex, and counterstained with hematoxylin.
Statistical analysis
All numerical data are presented as means ± standard deviation. Differences between groups on D18 and D21 were tested by using an unpaired Student’s or Welch’s t test when the data had normal distribution or Mann–Whitney U test when the data deviated from normal distribution. Statistical significance was accepted at p values of less than 0.05.
Results
Relative mRNA expression levels of Smad1 and Wif1 in fetal rat lungs on D18 and D21
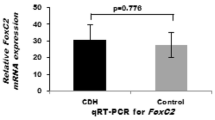
The relative mRNA expression levels of pulmonary Smad1 on D18 and D21 were significantly decreased in the nitrofen group (4.39 ± 1.99 and 1.66 ± 0.74, respectively) compared to control group (7.94 ± 1.06 and 3.16 ± 1.51, respectively) (p < 0.01) (Fig. 1). Pulmonary expression levels of Wif1 gene on D18 and D21 were also significantly downregulated in nitrofen group (4.73 ± 4.56 and 4.22 ± 3.06, respectively) compared to control group (8.67 ± 4.11 and 7.73 ± 2.57, respectively) (Fig. 2).
Protein expression of Smad1 and Wif1 in fetal rat lungs on D18 and D21
To determine whether the quantitatively decreased amounts of Smad1 and Wif1 transcripts were reflected in the qualitative amount of the proteins themselves in the nitrofen-induced hypoplastic lung, immunohistochemical studies were performed on D18 and D21.
In D18 control lungs, Smad1 is expressed strongly in the lung epithelium and weakly in the underlying mesenchyme (Fig. 3a). In contrast, in D18 CDH lungs, Smad1 immunoreactivity was markedly decreased in the epithelium and also in the mesenchyme (Fig. 3b). In D21 CDH lungs, Smad1 immunoreactivity was markedly diminished in the overall intensity (Fig. 3d), although strong Smad1 expression was seen in both epithelial and mesenchymal tissue of control lungs (Fig. 3c).
Smad1 and Wif1 immunohistochemistry on D18 and D21. On D18, Smad1 immunoreactivity was diminished in the epithelium and mesenchyme in CDH lungs (b) compared to control lungs (a). On D21, the overall intensity of Smad1 expression was decreased in CDH lung (d) compared to control lungs (c). On D18, Wif1 expression was decreased in the epithelium in CDH lungs (f), whereas strong expression was seen in the epithelium in control lungs (e). On D21, Wif1 immunoreactivity was decreased in the overall intensity in CDH lungs (h) compared to control lungs (g)
The Wif1 expression on D18 in CDH was decreased in the epithelial cells (Fig. 3f) compared to control lungs (Fig. 3e). In D21 CDH lungs, the overall immunoreactivity of Wif1 was diminished (Fig. 3h), whereas strong Wif1 expression was seen in control lungs (Fig. 3g).
Discussion
Nitrofen-induced pulmonary hypoplasia (PH) in the rat model has been widely used to investigate pathogenesis of PH associated with CDH. Although, recent studies from our laboratory has showed altered pulmonary expression of various genes in the nitrofen-induced PH [6–8], the precise molecular mechanisms by which nitrofen acts in this model are still not clearly understood.
It has been reported that BMP signaling plays a critical role in the regulation of cell differentiation during lung morphogenesis [14] and vasculogenesis [15]. Smad proteins are intracellular signaling molecules and some of which are putative transcription factors that transduce signals elicited by members of the transforming growth factor beta (TGF-β) superfamily [10]. It has been shown that the expression level of Smad 2, 3, 4 and 7 were markedly decreased in nitrofen-exposed lung [16]. Smad1, a member of Smad proteins, acts as a signal transducer in the BMP pathway [9, 10]. Smad1 is particularly in peripheral airway epithelial cells of early embryonic lung tissue [9]. In fetal lung development, it has been reported Smad1 protein expression in mesenchymal cells is increased during mid-late gestation, indicating that Smad1 plays a key role in the various aspects of late lung morphogenesis [9].
In the present study we observed that the pulmonary Smad1 mRNA levels as well as Smad1 immunoreactivity was significantly decreased in the nitrofen group on D18 and D21 compared to controls.
Our findings are consistent with the observations by Xu et al. [13], who reported lung-specific abrogation of Smad1 in developing mouse lung epithelial cells causing respiratory distress and lethality due to abnormal fetal lung formation. Smad1 knockout mice have shown no differences in early branching morphogenesis. However, later in gestation, Smad1 knockout lungs displayed a decreased by dilation of the lumen and number of terminal sacs, enlarged saccular lumen and thickened mesenchymal tissues pathologically. Interestingly, the morphological abnormalities in this knockout model become most obvious at the end of gestation [13].
In Smad1 knockout mice, important signaling factors of lung morphogenesis such as Wnt inhibitor factor-1 (Wif1) are downregulated [13]. Wif1 expressed in normal epithelial cell of the lung, is a member of secreted proteins [17], bound to Wnt proteins, and inhibits their activity [18]. It remains unknown whether Wif1 inhibits the canonical, non-canonical Wnt pathway or both [19]. Studies from our laboratory have previously demonstrated that gene expression of a noncanonical Wnts, Wnt5a, is upregulated in the nitrofen-induced hypoplastic lung in the late gestation [20], suggesting that activating of noncanonical Wnt signaling may contribute to PH by disruption alveolar maturation [20]. In the present study, we observed downregulation of Wif1 in the nitrofen-induced hypoplastic lung on D18 and on D21.
In this study, we showed for the first time that pulmonary gene expression of Smad1 and Wif1 was significantly downregulated in the nitrofen CDH rat model compared to controls both on D18 and on D21. Taken together, these data suggest that downregulation of Smad1 and Wif1 signaling pathway may disrupt lung development during saccular stage and result in pulmonary hypoplasia in the nitrofen rat model of CDH. Further studies of Smad1 and Wif1 signaling in the nitrofen-induced hypoplastic lung are required and should provide insights into the pathogenesis of altered lung morphogenesis in CDH.
References
Gosche JR, Islam S, Boulanger SC (2005) Congenital diaphragmatic hernia: searching for answers. Am J Surg 190:324–332
Keijzer R, Puri P (2010) Congenital diaphragmatic hernia. Semin Pediatr Surg 19:180–185
Colvin J, Bower C, Dickinson JE et al (2005) Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 116:e356–e363
Yang W, Carmichael SL, Harris JA et al (2006) Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989–1997. Birth Defects Res 76:170–174
Kluth D, Kangah R, Reich P et al (1990) Nitrofen-induced diaphragmatic hernias in rats: an animal model. J Pediatr Surg 25:850–854
Doi T, Shintaku M, Dingemann J et al (2011) Downregulation of Midkine gene expression and its response to retinoic acid treatment in the nitrofen-induced hypoplastic lung. Pediatr Surg Int 27:199–204
Doi T, Sugimoto K, Ruttenstock E et al (2011) Prenatal treatment with retinoic acid activates parathyroid hormone-related protein signaling in the nitrofen-induced hypoplastic lung. Pediatr Surg Int 27:47–52
Dingemann J, Doi T, Ruttenstock E et al (2010) Abnormal platelet-derived growth factor signaling accounting for lung hypoplasia in experimental congenital diaphragmatic hernia. J Pediatr Surg 45:1989–1994
Chen C, Chen H, Sun J et al (2005) Smad1 expression and function during mouse embryonic lung branching morphogenesis. Am J Physiol Lung Cell Mol Physiol 288:1033–1039
Dick A, Risau W, Drexler H (1998) Expression of Smad1 and Smad2 during embryogenesis suggests a role in organ development. Dev Dyn 211:293–305
Takayasu H, Nakazawa N, Montedonico S et al (2007) Down-regulation of Wnt signal pathway in nitrofen-induced hypoplastic lung. J Pediatr Surg 42:426–430
Alejandre-Alcázar MA, Shalamanov PD, Amarie OV et al (2007) Temporal and spatial regulation of bone morphogenetic protein signaling in late lung development. Dev Dyn 236:2825–2835
Xu B, Chen C, Chen H et al (2011) Smad1 and its target gene Wif1 coordinate BMP and Wnt signaling activities to regulate fetal lung development. Development 138:925–935
Masterson JC, Molloy EL, Gilbert JL et al (2011) Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal 23:398–406
Southwood M, Jeffery TK, Yang X et al (2008) Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol 214:85–95
Leinwand MJ, Zhao J, Warburton D (2002) Murine nitrofen-induced pulmonary hypoplasia does not involve induction of TGF-β signaling. J Pediatr Surg 37:1123–1127
Wissmann C, Wild PJ, Kaiser S et al (2003) Wif1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 201:204–221
Hsieh JC, Kodjabachian L, Rebbert ML et al (1999) A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398:431–436
Lin YC, You L, Xu Z et al (2007) Wnt inhibitory factor-1 gene transfer inhibits melanoma cell growth. Hum Gene Ther 18:379–386
Doi T, Puri P (2009) Up-regulation of Wnt5a gene expression in the nitrofen-induced hypoplastic lung. J Pediatr Surg 44:2302–2306
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujiwara, N., Doi, T., Gosemann, JH. et al. Smad1 and WIF1 genes are downregulated during saccular stage of lung development in the nitrofen rat model. Pediatr Surg Int 28, 189–193 (2012). https://doi.org/10.1007/s00383-011-2987-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-011-2987-0