Abstract
Aim
To perform a definitive procedure in pure esophageal atresia by gastric pull-up in the newborn.
Patients/methods
A primary gastric pull-up was performed in six newborns with pure esophageal atresia that presented between 1998 and 2009. The cervical esophagus was mobilized through the neck, the stomach was mobilized through laparotomy, the left gastric artery was ligated, and Pyloromyotomy was done. The stomach was brought into the neck via the trans hiatal route. A single-layer esophageo-gastric anastomosis was done in the neck in all.
Results
The mean birth weight was 2.1 kg (range 1.9–2.7) and the age at surgery varied from 3 to 7 days (mean 4.5 days). The mean operative time was 146 min. All six neonates received postoperative elective ventilation for a period of 2–7 days (mean 5.3). Epidural morphine was given for postoperative pain relief. Four received TPN for 5–13 days. Three had minor leaks from the neck wound that healed spontaneously. Mean hospital stay was 14.6 with a range 13–20 days. There was no mortality.
Conclusion
It is feasible to perform the gastric pull-up for long gap esophageal atresia in the newborn period, as a definitive procedure with no added risks to life in experienced hands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common initial procedure adopted in neonatal period for pure esophageal atresia is a cervical esophagostomy and an abdominal gastrostomy followed by an esophageal substitution at around 1 year of age. Till date, performing an esophageal replacement in an infant has been reported to be associated with a high rate of complications and mortality in the limited experience reported worldwide.
Adopting the best method for the esophageal substitution at a tender age does not have much to debate. It is vital to remember that the neonate does not allow a second attempt for a failed graft uptake. Though jejunal free grafts offer several theoretical advantages such as appropriate caliber and retained peristalsis, it is a technically demanding procedure, especially, in the newborns. The graft may fall short, and the cumulative experience with the procedure in children remains limited, with none reported in newborns on review of literature [1].
Though colonic interposition has occasionally been performed in infants less than 3 months of age, but the main concern is the precarious blood supply of the colon in the neonates predisposing to gangrene and perforation if placed in the mediastinum in the presence of severe sepsis [2, 3]. The neonatal stomach is too small to allow the reconstruction of a gastric tube. Thus, the gastric transposition procedure has the least short-term morbidity and mortality as compared with the alternative procedures.
The surgical technique adopted for primary gastric transposition in the neonatal period requires expertise that can be attained by performing the procedure in older children first. The feasibility and the technical expertise required are discussed.
Patients and methods
Aim
To perform a definitive procedure by bridging the wide gap in pure esophageal atresia by gastric pull-up in the newborn as a definitive surgical procedure to avoid the prolonged hospitalization in commonly available procedures.
Patients/methods
Primary gastric pull-up was performed in six newborns presented with pure esophageal atresia and a wide gap, between 1998 and 2009.
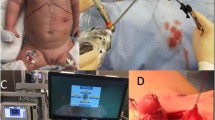
Baby under general anesthesia was placed in the supine position with a roll under the neck. The head was turned to the left with the endotracheal tube (ETT) fixed around the left angle of the mouth. The cervical esophagus was mobilized through a neck incision on the right side. Through the upper abdominal midline incision, the stomach was mobilized. The left gastric vessels were ligated away from the stomach. Short gastric vessels were ligated. The gastroepiploic arcade and the right gastric vessels were preserved. The spleen was preserved. The highest point in fundus was identified for anastomosis. The esophageal stump was transected and the defect was closed in two layers. As bilateral vagotomies invariably accompany while delivering the lower esophageal pouch, a drainage procedure in the form of pyloromyotomy was done to avoid gastric stasis.
Kocher manoeuvre was performed on the second part of duodenum to gain length and mobility. Pyloromyotomy was done. A two- to three-finger breadth transhiatal posterior mediastinal tunnel was created between the membranous posterior surface of trachea and the pre-vertebral fascia. Stomach was taken up to the neck through the esophageal hiatus, ensuring that there was no torsion of the stomach during pull-up. A single-layer esophagogastric anastomosis was made at the highest point of the stomach using 5–0 polyglactin sutures. The neck wound was drained by a glove drain.
Postoperative vital sign monitoring and elective ventilation with paralysis was provided to all. A nasogastric tube was used to prevent acute gastric dilatation. Relief of postoperative pain was managed by morphine infusion through the epidural catheter. Small frequent oral feeds were instituted usually from fifth day onwards or as was found feasible, in a head up position.
Results
The mean birth weight was 2.1 kg (range 1.9–2.7) and the age at surgery varied from 3 to 7 days (mean 4.5 days). A single-layer, primary anastomosis was done in all, in the neck. The mean operative time was 146 min. All the 6 neonates received postoperative elective ventilation for a period varying from 2–7 days (mean 5.3). Four newborns received TPN for 5–13 days. Three developed postoperative minor leaks from the neck wound that healed spontaneously during the hospital stay. Mean hospital stay was 14.6 with a range 13–20 days. There was no mortality in this series.
Discussion
Gastric transposition is one of the main procedures used for replacing the esophagus for various reasons including the esophageal congenital malformations. It is considered as a reliable method for the esophageal replacement even in emergency situations with a good reported outcome [4–6].
The blood supply to the stomach is rich and remained in the subserosal plane without the risk of vessels getting thrombosed. The size of the stomach is also small, especially, if it is a case of pure EA. The gastric wall is also quite thick and muscular to withstand the mediastinal infection, if any. Thus, gastric transposition was considered, and it remained the only option to replace the esophagus in the neonatal period. The authors performed this procedure in newborns only after obtaining the initial successful experience of gastric transposition performed in infants and children [7–10].
The advantages include: (a) technically easy procedure, (b) muscular stomach with a reliable blood supply, (c) adequate length possible for anastomosis, (d) single anastomosis required in the neck, and (e) thoracotomy is not required.
The disadvantages of the thoracic stomach include: (a) bulk of stomach in mediastinum or thorax can theoretically cause respiratory distress and decrease venous return, (b) reflux is common and can cause aspiration pneumonitis, (c) gastric emptying may be delayed despite pyloromyotomy. There may also be rapid gastric emptying leading to dumping, and (d) loss of gastric reservoir function and its effect on growth and development.
The experience with esophageal replacement in the neonates is still limited though the procedure was first described in 1980 by Atwel and Harrison [11] in four neonates with pure esophageal atresia and in two with EA/TEF, resulting in a survival of two only. Subsequently, in 1994, Gomez [2] reported another survivor with gastric pull up performed for pure esophageal atresia, on day 13 of life.
The challenges posing concerns were that the newborns may (a) not withstand such a major surgical procedure, (b) develop an increased risk of respiratory and cardiac complications, and (c) have an increased risk of aspiration in the postoperative period as most of the conduits empty by gravity and not by active peristalsis. Our own experience and also that of others reported in the literature have proved this incorrect [6–9, 12]. Gastric pull is tolerated very well even in the newborn period in experienced hands, ensuring a good postoperative care, ventilatory support and pain relief.
The handling of the tissues is very important. From the experience of this procedure performed in infants and children, the authors realize that the short gastric vessels should not be diathermised, but should rather be ligated. Use of diathermy results in the spasm of the vessels and the fundal stomach becomes atonic and flabby, though, it has never resulted in ischaemic necrosis. It has always been feasible to bring the small sized stomach in pure esophageal atresia, up into the neck to perform an anastomosis after due mobilization including kocherization. The neonatal stomach in all cases easily adapted in the thoracic cavity without causing any respiratory embarrassment. An ideal situation should be that the baby should be around 2 kg in weight, with no chest infection, no major congenital heart disease and no multiple associated multiple anomalies. Pyloroplasty should not be done as it reduces the length of the stomach by a centimeter or so. A pyloromyotomy is a good alternative option sufficient to provide drainage. The trans hiatal route is quite feasible and short as well for pulling the stomach up in the neck. The tunnel can be created easily using fingers from above in the neck and below through the esophageal hiatus. This route was used in all the newborns in this series. Performing a primary gastric pull up in the neonatal period as an emergency procedure in pure esophageal atresia offered a definitive procedure and avoided the complications and high mortality associated with diversion (esophagostomy and gastrostomy).
The main advantage of performing such an operation in the newborn is that a thoracotomy is not required and there is no chance of any leak in the chest. Moreover, a neonatal gastric pull-up can be performed as a definitive procedure to avoid esophageal diversion, repeated surgical procedures requiring prolonged hospital stay/visits. The stomach is very vascular and muscular. It can safely be used even in the presence of mediastinal sepsis that usually follows the major leaks after primary anastomosis for EA/TEF. Gastric pullup can be performed even in emergency without the need for any preparation. The absence of mortality in this series was encouraging.
The long term concerns related to the gastric clearances, biliary reflux, anemia, biliary gastritis and achlorhydria and the loss of function of the gastric reservoir affecting the growth and development of the patient, still remain unanswered. However, the long term results following the use of stomach in infants and children in the authors experience have been very encouraging with no serious disability.
To conclude, gastric transposition may be considered as one of the definitive procedures to replace the esophagus even in the newborns for pure esophageal atresia with long gap, to avoid other procedures requiring repeated surgeries and the hospital visits.
References
Spicer RD, Cusick EL (1996) Esophageal substitution by jejunal free graft: follow up data and an evaluation. Pediatr Surg Int 11:227–229
Vargas Gomez M (1994) Esophageal replacement in patients under 3 months of age. J Pediatr Surg 29:487–491
Hernandez F, Rivas S, Avila LF, Luis AL, Martinez L, Lassaletta L, Murcia FJ, Tovar JA (2003) Early esophageal replacement in patients with esophageal atresia. Cir Pediatr 16:112–115
Spitz L (1996) Gastric transposition for esophageal replacement. Pediatr Surg Int 11:218–220
Spitz L, Kiely E, Pierro A (2004) Gastric transposition in children—a 21-year experience. J Pediatr Surg 39:276–281
Davenport M, Hosie GP, Tasker RC, Gordon I, Kiely EM, Spitz L (1996) Long-term effects of gastric transposition in children: a physiological study. J Pediatr Surg 31:588–593
Gupta DK, Bajpai M, Kataria R (1997) Esophageal replacement by stomach in children. Eur J Pediatr Surg 7:143–146
Gupta DK, Sharma S, Arora MK et al (2007) Esophageal replacement in the neonatal period in infants with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg 42:1471–1477
Gupta DK, Srinivas M, Agarwala S et al (2003) Neonatal gastric pull up: reality or myth? Pediatr Surg Int 19:100–103
Gupta DK, Charles AR, Srinivas M (2004) Manometric evaluation of the intrathoracic stomach after gastric transposition in children. Pediatr Surg Int 20:415–418
Atwell JD, Harrison GS (1980) Observations on the role of esophagogastrostomy in infancy and childhood with particular reference to the long-term results and operative mortality. J Ped Surg 15:303–309
Spitz L (2009) Gastric transposition in children. Semin Pediatr Surg 18:30–33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Gupta, D.K. Primary gastric pull-up in pure esophageal atresia: technique, feasibility and outcome. A prospective observational study. Pediatr Surg Int 27, 583–585 (2011). https://doi.org/10.1007/s00383-010-2835-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-010-2835-7