Abstract
Purpose
We have studied whether curcumin protects different pulmonary aspiration material-induced lung injury in rats.
Materials and methods
The experiments were designed in 60 Sprague–Dawley rats, randomly allotted into one of six groups (n = 10): normal saline (NS, control), enteral formula (Biosorb Energy Plus, BIO), hydrochloric acid (HCl), NS + curcumin-treated, BIO + curcumin-treated, and HCl + curcumin-treated. NS, BIO, HCl were injected in to the lungs. The rats received curcumin twice daily only for 7 days. Seven days later, both lungs in all groups were examined histopathologically, immunohistochemically, and biochemically. Histopathologic examination was performed according to the presence of peribronchial inflammatory cell infiltration, alveolar septal infiltration, alveolar edema, alveolar exudate, alveolar histiocytes, interstitial fibrosis, granuloma, and necrosis formation. Immunohistochemical assessments were examined for the activity of inducible nitric oxide synthase (iNOS) and the expression of surfactant protein D (SP-D). Malondialdehyde (MDA), hydroxyproline (HP), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activity were measured in the lung tissue.
Results
Our findings show that curcumin inhibits the inflammatory response reducing significantly (P < 0.05) all histopathological parameters in different pulmonary aspiration models. Pulmonary aspiration significantly increased the tissue HP content, MDA levels and decreased the antioxidant enzyme (SOD, GSH-Px) activities. Curcumin treatment significantly decreased the elevated tissue HP content, and MDA levels and prevented inhibition of SOD, and GSH-Px enzymes in the tissues. Furthermore, our data suggest that there is a significant reduction in the activity of iNOS and a rise in the expression of SP-D in lung tissue of different pulmonary aspiration models with curcumin therapy.
Conclusion
Our findings support the use of curcumin as a potential therapeutic agent in acute lung injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pulmonary aspiration of gastric contents while being a common event, represents a serious complication of a wide variety of clinical disorders. Aspiration of gastric contents continues to be a concern for physicians involved in the care of critically ill patients [1, 2]. Gastric content aspiration is one of the major causes of acute lung injury. The severity of the histopathologic changes results in lung injury based on several factors such as pH value, volume, content of the aspirated material, and the response of the patients [3].
Different animal models have been developed to investigate the mechanisms, characteristics, and pathophysiology of lung injury [4]. Even though the mechanism of lung injury is not accurately comprehended there is evidence for the implication of oxidative damage by reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are increasingly regarded as key substances modulating the pulmonary vascular endothelial damage that characterized acute respiratory distress syndrome (ARDS). The resulting vascular endothelial damage is accountable for the principal clinical manifestations of ARDS [5–7]. There is convincing evidence that ROS play a major role in mediating injury to the endothelial barrier of the lung in the presence of endotoxin or sepsis. ROS are capable of reacting with cellular lipids, proteins, and nucleic acids leading to changes in the structure and function of alveolar cells. This condition is supported by several animal studies. In these studies, antioxidant therapy has been beneficial in the protection and the treatment of lung injury [5–7].
Surfactant protein D (SP-D) is a member of the collectin family of proteins, which play important roles in innate host defense of the lung and regulation of surfactant homeostasis and is synthesized in alveolar type II cells and Clara cells of lungs [4]. Alveolar cell damage leads to decreased and impaired synthesis, secretion, function, and composition of SP-D in acute lung injury, [8, 9]. Therewithal, little knowledge presents about the histopathological benefits of antioxidants on the development of different gastric content induced lung injury [10].
Curcumin, a widely used orange-yellow curry pigment from turmeric (Curcuma longa), has been designated to be a forceful anti-inflammatory, anti-cancer and anti-oxidant agent, and is under preclinical trial for cancer prevention and anti-inflammation [11, 12]. Lately, curcumin was shown to inhibit the production of nitric oxide (NO) and the expression of inducible NO synthase (iNOS) [13]. Overproduction of NO and oxidants seem to be important factors in the pathology of the inflammatory process in lung injury. Inhibition of iNOS and protection of oxidant effect have been effective in reducing tissue damage in several models of inflammation [13–15].
According to our hypothesis, curcumin is beneficial in the protection of alveolar cell via increase of SP-D secretion, inhibition of iNOS, and prevention of oxidative damage. In order to explain our hypothesis, we examined the effects of curcumin treatment on lung injury due to different aspiration materials in rats.
Materials and methods
The Ethical Committee of Trakya University approved all animal procedures and the experimental protocol. Efforts were made to minimize animal suffering and reduce the number of animals used in experimental groups.
Animals
The experiments were performed in 60 Sprague–Dawley rats, ranging in weight from 230 to 260 g. Rats were provided by the Experimental Research Center of the Medical Faculty of Trakya University. The rats were kept in a windowless animal quarter where temperature (22 ± 2°C) and illumination were automatically controlled (light on at 7 a.m. and off at 9 p.m. 14 h light/10 h dark cycle). Humidity ranged from 50 to 55%. Animals were given free access to diet and water until the night before the experiment, when they were fasted.
Experimental protocol
Sixty animals were included in each of the following six groups (n = 10): normal saline (NS, control), enteral formula (Biosorb Energy Plus (BIO), Nutricia, Zoetermeer, The Netherlands), Hydrochloric acid (HCl 0.1 N, pH 1.25), NS + curcumin-treated, BIO + curcumin-treated, and HCl + curcumin-treated. The rats were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally, and allowed to breathe spontaneously throughout the entire experimental protocol. The animals were placed in a supine position with the extremities pulled caudally to facilitate exposure of the trachea. The trachea was exposed through an anterior neck incision and a direct puncture with a 24-gauge needle on a 1-mL tuberculin syringe was performed two to four tracheal rings below the larynx. NS, BIO, HCl were injected into the lungs in a volume of 2 mL/kg. After instillation of NS, BIO, and HCl, the tuberculin syringe was removed, and the neck incision was repaired with a 6-0 Ethilon suture. Animals were observed until they recovered from anesthesia. After surgical procedure, the rats received curcumin twice daily (200 mg/kg per day, orally) only for 7 days. Seven days later, all rats were killed with intraperitoneal injection of ketamine hydrochloride; the trachea and both lungs were removed.
Histopathological procedures
Portions of right lung (anterior lobe, median lobe, posterior lobe, and post caval lobe) and left lung (upper left lobe, and lower left lobe) were individually immersed in 10% neutral-buffered formalin, dehydrated in alcohol, embedded in paraffin, and then cut into 5-μm thick cross-sections through the middle of the lobe so that each section included hilum to periphery. Sections were placed on slides, deparaffinated, and stained with hematoxylin and eosin (H&E) using standard procedures. Six slides were analyzed in a standardized fashion. Each lung lobe sections were divided equally for histopathological, immunohistochemical and biochemical investigations. Each slide was examined and evaluated in random order under blindfold conditions for immunostaining by a histologist (M.K.) and stained with H&E for histopathologic assessment under a standard light microscopy by a pathologist (Ö.Y.). The slides were examinedThe slides were examined for the presence of peribronchial inflammatory cell infiltration (PICI), alveolar septal infiltration (ASI), alveolar edema (AED), alveolar exudate (AEX), alveolar histiocytes (AHI), interstitial fibrosis (IF), granuloma (GRA), and necrosis (NEC) formation. These changes were scored according to the four-point scale used by Takil et al. [16]. A histopathological assessment was performed in at least randomly selected eight microscopic high-power fields from each lung lobe. The final score determined in each category for each individual animal was the mean of the scores from the sections of the lungs examined.
Immunohistochemistry
Immunocytochemical reactions were performed according to the ABC technique described by Hsu et al. [17]. The procedure involved the following steps: (1) endogenous peroxidase activity was inhibited by 3% H2O2 in distilled water for 30 min, (2) the sections were washed in distilled water for 10 min, (3) non-specific binding of antibodies was blocked by incubation with normal goat serum (DAKO X 0907, Carpinteria, CA) with PBS, diluted 1:4, (4) the sections were incubated with specific rabbit polyclonal anti-iNOS antibody (Cat. # RB-1605-P, Neomarkers, USA) and specific rabbit polyclonal anti-surfactant protein D (SP-D) antibody (Cat. # AB3434, Chemicon, USA), diluted 1:50 for 1 h, and then at room temperature, (5) the sections were washed in PBS 3 × 3 min, (6) the sections were incubated with biotinylated anti-mouse IgG (DAKO LSAB 2 Kit), (7) the sections were washed in PBS 3 × 3 min, (8) the sections were incubated with ABC complex (DAKO LSAB 2 Kit), (9) the sections were washed in PBS 3 × 3 min, (10) peroxidase was detected with an aminoethylcarbazole substrate kit (AEC kit; Zymed Laboratories), (11) the sections were washed in tap water for 10 min and then dehydrated, (12) the nuclei were stained with hematoxylin, and (13) the sections were mounted in DAKO paramount.
The positive immunostaining of iNOS and SP-D cell numbers were scored in a semiquantitative manner in order to determine the differences between the control group and the experimental groups in the distribution patterns of intensity of immunolabeling of lung tissue. The numbers of the positive staining were recorded as weak (±), mild (+), moderate (++), strong (+++) and very strong (++++). This analysis was performed in at least randomly selected eight microscopic high-power fields from each lung lobe section, in two sections from each animal at 400× magnification. The final score determined in each category for each individual animal was the average of the scores from the sections of the lungs examined.
Biochemical procedures
At the end of the experiment, rats in all groups were starved overnight for 12 h. After killing, the harvested lung tissues samples were quickly washed in cold saline and stored at −70°C.
Measurement of tissue hydroxyproline
Total hydroxyproline (HP) content of the lung tissue samples was measured as an assessment of lung collagen content. A spectrophotometric assay was used to quantify lung HP [18]. Briefly, the lung was removed from the −70°C freezer and homogenized in 5% trichloroacetic acid (1:9 wt/vol). The homogenized samples were centrifuged for 10 min at 4,000g, and the pellet was washed twice with distilled water and then hydrolyzed for 16 h at 100°C in hydrochloric acid (6 N HCl). The HP level was expressed as micrograms per milligram wet lung tissue.
Measurement of tissue malondialdehyde level
Lung tissue samples were frozen at −70°C and irrigated well with a solution of sodium chloride (NaCl) (0.9%). By admixing it with potassium chloride (KCl) (1.5%), homogenization at a ratio of 1:10 was achieved. The DIA×9000 Homogenizer (Heidolph Instruments, Germany) was used to homogenize the tissue samples. The lipid peroxide level in the centrifuged tissue homogenates was measured according to the method described by Ohkawa et al. [19]. The reaction product was assayed spectrophotometrically (Shimadzu UV-1700, Japan) at 532 nm. The lipid peroxide level was expressed as the nanomole (nmol) of malondialdehyde (MDA) per milligram of lung tissue protein. Protein levels were measured according to the method described by Lowry et al. [20].
Measurement of tissue superoxide dismutase activity
Superoxide dismutase (SOD) activity was determined according to the method of Sun et al. [21]. This method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the XO system as a superoxide generator. Activity was assessed in the ethanol phase of the lysate after 1.0 ml ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the NBT reduction rate. SOD activity was also expressed as units per milligram protein.
Glutathione peroxidase activity
Glutathione peroxidase (GSH-Px) activity was measured by the method of Paglia et al. [22]. The enzymatic reaction was initiated in a tube containing the following items: NADPH, reduced glutathione (GSH), sodium azide, and glutathione reductase by addition of H2O2 and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was given in units per gram protein. All samples were assayed in duplicate.
Statistical analysis
All statistical analyses were completed using SPSS statistical software (SPSS for Windows, version 11.0). The data of biochemical findings were presented in mean (±) standard deviation (SD) and dual comparisons between groups exhibiting significant values were evaluated with Kruskall Wallis test followed by Mann–Whitney U test. The degrees of histopathological severity were analyzed with one-way analysis of variance and Tukey test for detection of significant differences between groups. All data for histopathological grades are expressed in a dot-scatter plot. These differences were considered significant when probability was less than 0.05.
Results
All animals survived until being killed at 7 days. No animal were excluded from analysis. All animals were examined immunohistochemically, biochemically, and histopathologically for lung injury.
Biochemical findings
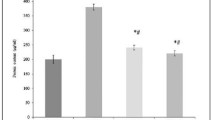
The values of the tissue HP content, MDA levels, SOD and GSH-Px activities, and statistical differences of these measurements are shown in Table 1. Pulmonary aspiration significantly increased the tissue HP content, MDA levels and decreased (P < 0.05) the antioxidant enzyme (SOD, GSH-Px) activities (Table 1). Curcumin treatment significantly (P < 0.05) decreased the elevated tissue HP content, and MDA levels and prevented inhibition of SOD, and GSH-Px (P < 0.05) enzymes in the tissues (Table 1).
Histopathological findings
The body weights of animals were similar across groups (P > 0.05). Histopathological results of study groups are presented in Figs. 1 and 2. Histopathological parameters including PICI, ASI, AED, AEX, AHI, IF, GRA, and NEC were decreased in all groups treated with curcumin compared to untreated groups. PICI was observed in all groups, but was statistically severer in group HCl than group BIO (P < 0.01) and NS (P < 0.001). There was statistical difference between groups BIO and NS (P < 0.05). In the curcumin treated (Fig. 1) rat lungs, the PICI was significantly less than in the only HCl treated groups (P < 0.01). ASI was statistically higher in groups BIO (P < 0.01), and HCl (P < 0.001) compared to NS group. Curcumin treatment did not significantly decrease ASI, except in HCl group (P < 0.01). AED, AEX, AHI, IF, GRA, and NEC were found significantly higher HCl compared to NS group (P < 0.001). Although curcumin treatment significantly decreased AED, AEX, AHI, IF, GRA, and NEC in HCl group, it had no significantly protective effects aforementioned findings in groups BIO and NS (Fig. 1).
All the data for histopathological grades are expressed in a dot-Scatter plot. Group 1 aspiration of normal saline solution (NS). Group 2 aspiration NS treated with circumin (NS + circumin). Group 3 aspiration of Biosorp (BIO). Group 4 aspiration of BIO treated with circumin (BIO + circumin). Group 5 aspiration of hydrochloric acid (HCl). Group 6 aspiration of HCl treated with curcumin (HCl + curcumin) (n = 10 pulmonary for each groups)
a Showing normal lung tissue morphology, b diffuse hemorrhagic area (asterisks) is showed in the necrotic lung tissue, c rat lung showing peribronchial inflammatory cell infiltration (asterisks), d rat lung showing alveolar septal infiltration (asterisks), e rat lung showing alveolar edema (asterisks), f rat lung showing alveolar exudate (asterisks), g showing necrotic lung tissue (asterisks) and multivacuolated alveolar histiocytes forming clusters in alveolar spaces (arrowhead), h showing granuloma (asterisks) in necrotic lung tissue (among arrowhead). A Alveol, B Bronchiole (H&E, scale bar 50 μm)
Immunohistochemical findings
The number of alveolar type II cells positive for SP-D was semiquantitatively lower in groups BIO, and HCl than group NS. However, SP-D expression of degenerative alveolar type II cells was considerably restricted in these groups than group NS. Curcumin treatment significantly increased the number and expression of SP-D reactivity in alveolar type II cells. The number of alveolar cells positive for iNOS was semiquantitatively higher in groups BIO, and HCl than group NS. Curcumin treatment significantly decreased the number of iNOS immunopositive cells (Table 2; Fig. 3).
Immunohistochemical expression of iNOS and SP-D in the lung tissue. (iNOS, a) slightly positive iNOS immunoreactivity in normal lung tissue, (iNOS, b) strongly positive iNOS immunoreactivity in necrotic lung tissue, (iNOS, c) moderately positive iNOS immunoreactivity in treated lung tissue. (SP-D, a) strong SP-D immunostaining was present in alveolar type II cells of normal lung tissue, (SP-D, b) weak SP-D expression in degenerative alveolar type II cells of necrotic lung tissue (asterisks), (SP-D, c) increase positive SP-D immunoreactivity in alveolar type II cells of treated lung tissue. A Alveol, B Bronchiole, arrows positive reactivity (H&E, scale bar 50 μm)
Discussion
The present investigation reports the effect of curcumin, an antioxidant, on different aspiration material-induced lung injury in rats.
The pulmonary aspiration of gastric contents while being a common event represents a serious complication of a wide variety of clinical disorders. It commonly occurs in critically ill patients with disturbance of consciousness due to drug overdose, cerebrovascular disease, or high-risk disease, and sometimes after aspiration of regurgitated gastric contents in patients with presence of a nasogastric tube, supine position, tracheal intubation and mechanical ventilation or impaired upper airway reflexes during induction of anesthesia, or recovery from it [23–25]. The severity of lung injury was based on several factors such as pH value, volume and content of the aspirated material, and the response of the patients [3]. We used volume, content and pH similar to that reported in the literature for pulmonary aspiration in rats [15, 16].
The aspiration of lipoid material following the accidental ingestion of enteral formulas is the most common cause of lipoid pneumonia and the severity of lipoid material aspiration induced-lung injury may depend on the lipid content [2, 16, 26]. The mechanism of lipoid material in lung tissue after aspiration is not completely known. Most authors have showed that vegetable oils are found in Biosorb Energy Plus which do not dissolve in lung tissue and act like a foreign body, whereas animal fats are hydrolyzed into free fatty acids, induce acute or chronic inflammation with NEC. However, the presence of histiocytes with lipid content after lipid aspiration is the standard diagnostic histopathological finding in lipoid pneumonia in children [16, 27]. As a clinic, it represents cough, increasing dyspnea and chest pain, together with alveolar infiltrates in the radiography and the previous accidental intake of a lipid substance and vomiting [24]. According to one study, the pulmonary histopathologic effects of aspiration of Impact were more severe PICI (greater than aspiration of Biosorb and Pulmocare), abundant AHI, and AED in comparison with aspiration of saline, even though Impact had the lowest lipid content of all studied formulas [16]. In addition, some authors believe that the high lipid content formula might not be a risk factor for lipid aspiration [16]. Our histopathological findings are consistent with their results concerning the effects of different content lipid formulas aspiration.
Aspiration of gastric materials brings about lung injuries ranging from mild, subclinical pneumonitis to severe, adult respiratory distress syndrome with associated high mortality. In the clinical setting, the composition of aspirated gastric material can vary considerably and may include low pH secretions, food particulate material of varying size, and bacteria from oropharyngeal and gastric flora [28, 29]. The content of aspiration materials is thought to be highly important in determining the severity of lung injury in patients. Recent studies have shown that acid aspiration pneumonitis often complicated by secondary bacterial pneumonia and, in about a third of cases, causes severe lung injury [30–32]. Acid aspiration leads to increased neutrophil oxidative metabolism, an event associated with lung leukosequestration, damage of the alveolar epithelium, principally alveolar type I and type II cells and permeability increase [33]. However, all histopathological parameters were observed in all groups, but were statistically severer in group HCl. Our findings support previous studies [30–32].
Most of organs may be protected from the damaging effects of the ROS by enzymatic and non-enzymatic antioxidant defence. These include enzymes like SOD, catalase, and GSH-Px [5–7]. MDA is a good indicator of free radical activity and its altitude presents increased lipid peroxidation [34]. However, alveolar macrophages can manufacture potent ROS such as superoxide radicals and consequently peroxynitrite which can be produced by the reaction of NO with superoxide radicals and exhibits a highly oxidative species [5–7]. ROS play major role in mediating damage to the endothelial barrier of the lung in the sepsis. These species are produced by activated alveolar macrophages, stimulated endothelial cells, and damaged structure, and function of alveolar cells [5, 6]. In addition, several animal models have been developed to investigate the mechanisms, characteristics, and pathophysiology of aspiration lung injury and some proinflammatuar cytokines have been implicated in the development of lung injury [31]. A few studies have shown that some inflammatory factors associated with lung injury, such as neutrophil-mediated ROS and proinflammatory cytokines [35–37]. Antioxidant therapy has been useful in the protection and the treatment of lung injury in some animal studies [5–7]. In one study curcumin has been shown to reduce the total lung HP, suppress alveolar macrophage production of TNF-a, superoxide and NO and contribute to the decreased lung collagen in BLM-treated animals. They believed that curcumin might be an antifibrotic and antiinflammatory compound for BLM-induced pulmonary fibrosis in rats [12]. In our study, curcumin which has been found to possess antioxidant, anti-inflammatory, anticancer, and other treatment activities diminished HP accumulation and MDA levels and prevented inhibition of SOD, and GSH-Px enzymes in the tissues due to acid instillation. This finding consistent with the results of Punithavathi et al. [12] concerning the effects of BLM-induced lung fibrosis.
Nitric oxide is a highly reactive radical synthesized from l-arginine by the action of NOS and can be derived by almost all mammalian cells, including endothelium lining the vasculature, neurones of the central and enteric nervous system, and immune system cells [38, 39]. An iNOS enzyme whose expression in endothelium, epithelium, and inflammatory cells requires protein synthesis, is induced by cytokines and lipopolysaccharide, and produces large amounts of NO for extended periods of time [40]. For inflammatory reactions that lead to injury, the role of NO is controversial, with evidence for proinflammatory as well as antiinflammatory effects [41]. However, little knowledge exists about the pharmacological benefits of NOS inhibitors on the development of acid-induced lung injury. In previous studies, aspirations of gastric components were lead to activation of macrophages and release of proinflammatory cytokines such as NO. In their animal models selective iNOS inhibition was prevented lung injury and a significant increase in the metabolism of NO, nitrite and nitrate was also observed in bronchoalveolar lavage fluid of acid-instilled rats or canines [31, 41–45]. Our data suggest that there is a significant increasing in the activity of iNOS in lung tissue of different aspiration materials such as lipid content formula (BIO), and acid (HCl).
Acute lung injury is a frequent cause of morbidity and mortality following pulmonary disease or systemic infections. Injury to the alveolar epithelial barrier is major determinant of the extent and severity of clinical acute lung injury [8, 46]. SP-D is a member of the collectin family of proteins, which play important roles in innate host defense of the lung and regulation of surfactant homeostasis [47–49]. SP-D is synthesized in alveolar type II cells and Clara cells of rat lungs and is an inhibitor of lipid peroxidation and oxidative cell damage. SP-D binds directly to alveolar macrophages and leukocytes and exerts a potent chemotactic effect on neutrophils and monocytes during pulmonary infection disease [4]. In addition, alveolar cell damage leads to decreased and impaired synthesis, secretion, function, and composition of SP-D in acute lung injury [8, 9]. Some authors reported that SP-D enters circulation primarily from the lung with alveolar damage and serum SP-D is a marker of lung injury in rats [50]. There are only few reports of the role of SP-D in lung injury due to gastric materials [10].
In conclusion, our results indicate that curcumin exhibits an obvious protective effect to oxidative alveolar cell damage by increasing SP-D secretion and reduction of iNOS activity. These findings support the use of curcumin as a potential therapeutic agent in acute lung injury.
References
Tasch MD, Stoelting RK (1996) Aspiration prevention, prophylaxis, and traetment. In: Benumoff JL (ed) Airway management. Mosby, St Louis, pp 183–201
Shepherd KE, Faulkner CS, Thal GD, Leiter JC (1995) Acute, subacute, and chronic histologic effects of simulated aspiration of a 0.7% sucralfate suspension in rats. Crit Care Med 23:532–536. doi:10.1097/00003246-199503000-00019
James CF, Modell JH, Gibbs CP, Kuck EJ, Ruiz BC (1984) Pulmonary aspiration: effects of volume and pH in the rat. Anesth Analg 63:665–668
Leth-Larsen R, Nordenback C, Tornoe I, Moeller V, Schlosser A, Koch C et al (2003) Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia. Clin Immunol 108:29–37. doi:10.1016/S1521-6616(03)00042-1
Bhatia M, Moochhala S (2004) Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202(2):145–156. doi:10.1002/path.1491
Tasaka S, Amaya F, Hashimoto S, Ishızaka A (2008) Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10(4):739–753. doi:10.1089/ars.2007.1940
Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W (1999) Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med 25(2):180–185. doi:10.1007/s001340050813
Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA (2003) Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 31:20–27. doi:10.1097/00003246-200301000-00003
Herbein JF, Wright JR (2001) Enhanced clearance of surfactant protein D during LPS-induced acute inflammation in rat lung. Am J Physiol Lung Cell Mol Physiol 281:L268–L277
Guzel A, Başaran U, Aksu B, Kanter M, Yalcın O, Aktas C, Guzel A, Karasalıhoglu K (2008) Protective effects of S-methylisothiourea sulfate on different aspiration materials-induced lung injury in rats. Int J Pediatr Otorhinolaryngol 72(8):1241–1250. doi:10.1016/j.ijporl.2008.05.001
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10(3):511–545. doi:10.1089/ars.2007.1769
Punithavathi D, Venkatesan N, Babu M (2000) Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol 131:169–172. doi:10.1038/sj.bjp.0703578
Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY et al (2008) Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomed Pharmacother (Feb):20 (in press)
Mlsko TP, Moore WM, Kasten TP, Nlckols GA, Corbett JA, Tilton RG (1993) Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol 233:119–125. doi:10.1016/0014-2999(93)90357-N
Jian MY, Koizumi T, Kubo K (2005) Effects of nitric oxide synthase inhibitor on acid aspiration-induced lung injury in rats. Pulm Pharmacol Ther 18:33–39. doi:10.1016/j.pupt.2004.07.007
Takil A, Umuroglu T, Gogus YF, Etı Z, Yildizeli B, Ahiskali R (2003) Histopathologic effects of lipid content of enteral solutions after pulmonary aspiration in rats. Nutrition 19:666–669. doi:10.1016/S0899-9007(03)00057-1
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Kivirikko KI, Laitinen O, Prockop DJ (1967) Modifications of a specific assay for hydroxyproline in urine. Anal Biochem 19:249–255. doi:10.1016/0003-2697(67)90160-1
Ohkawa H, Oshishi N, Yagi K (1979) Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Lowry OH, Rosenbrough NJ, Farr AC, Randall RJ (1951) Protein measurement with the folin phenol regent. J Biol Chem 193:265–275
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pawlik MT, Schreyer AG, Ittner KP, Selig C, Gruber M, Feuerbach S et al (2005) Early treatment with pentoxifylline reduces lung injury induced by acid aspiration in rats. Chest 127:613–621. doi:10.1378/chest.127.2.613
Metheny NA, Clouse RE, Chang YH, Stewart BJ, Oliver DA, Kollef MH (2006) Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit Care Med 34:1007–1015. doi:10.1097/01.CCM.0000206106.65220.59
DeLegge MH (2002) Aspiration pneumonia: incidence, mortality and at-risk populations. JPEN J Parenter Enteral Nutr 26(6):S19–S24
Garzón Lorenzo P, Torrent Vernetta A, Server Salvà L, de Vicente CM, García-Cendón C, Gartner S (2008) Exogenous lipoid pneumonia. An Pediatr (Barc) 68(5):496–498. doi:10.1157/13120049
Ahrens P, Noll C, Kitz R, Willigens P, Zielen S, Hofmann D (1999) Lipid-laden alveolar macrophages: a useful marker of silent aspiration in children. Pediatr Pulmonol 28:8. doi:10.1002/(SICI)1099-0496(199909)28:19+<8::AID-PPUL1>3.0.CO;2-V
Hutson AD, Davidson BA, Raghavendran K, Chess PR, Tait AR, Holm BA et al (2006) Statistical prediction of the type of gastric aspiration lung injury based on early cytokine/chemokine profiles. Anesthesiology 104:73–79
Hyers TM (1993) Prediction of survival and mortality in patients with the adult respiratory distress syndrome. New Horiz 1:466–470
Jian MY, Koizumi T, Tsushima K, Kubo K (2004) JTE-607, a cytokine release blocker, attenuates acid aspiration-induced lung injury in rats. Eur J Pharmacol 488:231–238. doi:10.1016/j.ejphar.2004.02.026
Kudoh L, Miyazaki H, Ohara M, Fukushima J, Tazawa T, Yamada H (2001) Activation of alveolar macrophages in acid-injured lung in rats: different effect of pentoxifylline on tumor necrosis factor-alpha and nitric oxide production. Crit Care Med 29:1621–1625. doi:10.1097/00003246-200108000-00020
Mıtsushima H, Oishi K, Ngao T, Ichinose A, Senba M, Iwasaki T et al (2002) Acid aspiration induces bacterial pneumonia by enhanced bacterial adherence in mice. Microb Pathog 33:203–210. doi:10.1006/mpat.2002.0529
Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB (1992) Reactive oxygen species and elastase mediate lung permeability after acid aspiration. J Appl Physiol 73(2):571–575
Aksu B, İnan M, Kanter M, Ozpuyan F, Uzun H, Durmuş G et al (2007) The effects of methylene blue on renal scarring due to pyelonepritis in rats. Pediatr Nephrol 22(7):992–1001. doi:10.1007/s00467-007-0464-8
Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V (1999) Regulation of neutrophil activation in acute lung injury. Chest 116:46S–54S. doi:10.1378/chest.116.suppl_1.46S-a
Davidson BA, Knight PR, Helinski JD, Nader ND, Shanley TP, Johnson KJ (1999) The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology 91:486–499. doi:10.1097/00000542-199908000-00024
Folkesson HG, Matthay MA, Hebert CA, Broaddus VC (1995) Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96:107–116. doi:10.1172/JCI118009
Moncada S (1992) The l-arginine:nitric oxide pathway. Acta Physiol Scand 145:201–227
Nathan C, Xie QW (1994) Nitric oxide synthases: roles, tolls and controls. Cell 78:915–918. doi:10.1016/0092-8674(94)90266-6
Gaston B, Drazen JM, Loscalzo J, Stamler JS (1994) The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149:538–551
Speyer CL, Neff TA, Warner RL, Guo RF, Sarma JV, Riedemann NC et al (2003) Regulatory effects of iNOS on acute lung inflammatory responses in mice. Am J Pathol 163:2319–2328
Bogdan C (2001) Nitric oxide and immune response. Nat Immunol 2:907–916. doi:10.1038/ni1001-907
Lee KH, Rico P, Billiar TR, Pinsky MR (1998) Nitric oxide production after acute, unilateral hydrochloric acid-induced lung injury in a canine model. Crit Care Med 26:2042–2047. doi:10.1097/00003246-199812000-00038
Harkin DW, Rubin BB, Romaschin A, Lindsay TF (2004) Selective inducible nitric oxide synthase (iNOS) inhibition attenuates remote acute lung injury in a model of ruptured abdominal aortic aneurysm. J Surg Res 120:230–241. doi:10.1016/j.jss.2004.03.011
Miyakawa H, Sato K, Shinbori T, Okamoto T, Gushima Y, Fujiki M et al (2002) Effects of inducible nitric oxide synthase and xanthine oxidase inhibitors on SEB-induced interstitial pneumonia in mice. Eur Respir J 19:447–457. doi:10.1183/09031936.02.00265902
Bachofen M, Weibel ER (1982) Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 3:35–56
Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J et al (1998) Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA 95:11869–11874. doi:10.1073/pnas.95.20.11869
Crouch EC (1998) Collectins and pulmonary host defense. Am J Respir Cell Mol Biol 19:177–201
Ikegami M, Scoville EA, Grant S, Korfhagen T, Brondyk W, Scheule RK et al (2007) Surfactant protein-D and surfactant inhibit endotoxin-induced pulmonary inflammation. Chest 132(5):1447–1454. doi:10.1378/chest.07-0864
Pan T, Nielsen LD, Allen MJ, Shannon KM, Shannon JM, Selman M et al (2002) Serum SP-D is a marker of lung injury in rats. Am J Physiol Lung Cell Mol Physiol 282:L824–L832
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzel, A., Kanter, M., Aksu, B. et al. Preventive effects of curcumin on different aspiration material-induced lung injury in rats. Pediatr Surg Int 25, 83–92 (2009). https://doi.org/10.1007/s00383-008-2282-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2282-x