Abstract
Purpose
In normal physiology, a vacuolar-type proton pump (V-ATPase) maintains an intracellular acid microenvironment in lysosome, endosome, and other endomembrane systems. Cancer cells overexpress V-ATPase compared with normal cells, and disturbances of the acid environment are thought to significantly impact the cancer cell infiltration and growth. Bafilomycin A1 (Baf-A1) is a specific inhibitor of the proton-pump inhibitor (PPI) V-ATPase. Neoplastic cells are reportedly more sensitive to Baf-A1 than normal cells, and the difference between the susceptibility to Baf-A1 in normal cells and that in cancer cells may become a target in the cancer therapy. With this in mind, we used cells of hepatoblastoma, the cancer type accounting for 80% of all childhood liver cancers, to investigate the effects of Baf-A1 as an inducer of cancer cell apoptosis and inhibitor of cancer cell reproduction
Methods and results
Electron microscopy showed significant morphological change of the hepatoblastoma cells of the Baf-A1-treated group compared with hepatoblastoma cells of the Baf-A1-free group. The rate of the apoptotic cell increased, and cell reproduction was inhibited. Moreover, the analysis of hepatoblastoma cells using the gene Chip gene expression analysis arrays showed that three of the 27 V-ATPase-related transcripts (ATP6V0D2, ATP6V1B1, and ATP6V0A1) were more weakly expressed in the Baf-A1-treated cells than in the Baf-A1-free cells. In normal human hepatic cells, on the other hand, the inhibition of cell growth of the Baf-A1-treated cells was negligible compared to that of the cells without Baf-A1 treatment. The result of apoptotic cell detection by morphological observations and flow cytometry revealed that Baf-A1 inhibits hepatoblastoma cellular reproduction by inducing apoptosis. On the other hand, the Baf-A1-conferred inhibition of cell growth was negligible in normal human hepatocytes
Conclusion
The V-ATPase inhibitor Baf-A1 has been proven to selectively inhibit the reproduction and induce the apoptosis of hepatoblastoma cells without adversely influencing normal hepatic cells. With these effects, V-ATPase inhibitors may hold promise as therapeutic agents for hepatoblastoma. Given that three V-ATPase-related genes (ATP6V0D2, ATP6V1B1, and ATP6V0A1) were more weakly expressed in the hepatoblastoma cells of the Baf-A1-treated group than in the Baf-A1-free cells, drug development targeting V-ATPase gene of hepatoblastomas is expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese Study Group for Pediatric Liver Tumor (JPLT), formed in Japan in 1991, has been administering a chemotherapy with an original protocol. The overall 2-year probability of survival of patients is comparable to the results from the West, at 81%. With advanced cancers, however, the rate of complete resection is 55% or less, no better than the results from the West, and the patients with unresectable cancers never survive without tumors. Even with this salvage therapy, the prognosis is very poor for patients with advanced cancers and patients with metastasis during treatment, and healing by the usual courses of chemotherapy is thought to be very difficult. The introduction of chemotherapy with newly developed drugs is indispensable.
Sporadic research in recent years has focused on tumor control using ATPase in new carcinostatic treatment strategies. V-ATPase is a multisubunit enzyme that belongs to ATP-dependent proton pumps, which are involved in a wide variety of physiological processes. V-ATPase mainly exists in ‘endomembrane systems’ such as lysosome and endosome. Because V-ATPase functions as an acidifier in the internal environments of endomembrane systems [1] and increases in various cancer cells, it could correlate well with cell differentiation, reproduction, and canceration.
Ohta et al. [2] found that V-ATPase is more highly expressed in pancreatic cancer cells than in normal cells, and that the expression levels increase in proportion to the grade of malignancy. These results suggest that disturbances of the acidic environment of carcinoma cells can have an important impact on the growth and functions of the cells. Selective inhibitor to V-ATPase is reported not only to induce cell differentiation by increasing pH, but also to inhibit the growth of various cells [3, 4] and induce apoptosis [5, 6]. Additionally, the susceptibility to the V-ATPase is known to be higher in the neoplastic cells than in normal cells. We speculate that this difference in the susceptibility to V-ATPase inhibitor in healthy cells and cancer cells may represent a potential target of cancer treatment.
We examined the prospects from medical treatment with proton pump (ATPase) for the cells of hepatoblastoma (HB), a common tumor accounting for 80% of all primary childhood liver cancers, and normal hepatocytes (human hepatocyte: HH). There have been no studies on the cell growth control (sensitive) for hepatoblastomas in relation to ATPase gene expression and the apoptosis-inducing effects of the V-ATPase inhibitor.
Materials and methods
Reagent adjustments
Bafilomycin A1 (Baf-A1) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) as a specific inhibitor of V-ATPase. Baf-A1 was dissolved with dimethylsulfoxide (DMSO, Wako Pure Chemical Industries, Ltd.) into a 10−5 M stock solution and kept in a freezer until use. In the Baf-A1-free group, the final concentration of DMSO was diluted with cell culture medium to a concentration of 0.1% or less. Reagents for culture additive were sterilized by filtration for the experiment after concentration adjustment with a 0.22 μm Millex GP Filter Unit (MILLIPORE, Corrigtwohill, Ireland).
Effect of bafilomycin A1 on human hepatoblastoma cells
Culture and adjustment of human hepatoblastoma cells
Human hepatoblastoma cells (HuH-6 Clone-5, well-differentiated type, JCRB0401) were purchased from the Health Science Research Resources Bank (Osaka, Japan). Based on the instructions of the supplier, the cells were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle medium with 10% fetal bovine serums (FBS) and 50 μg/ml gentamicin, and used for the tests upon reaching confluency.
Experimental groups
The experimental groups included hepatoblastoma cells cultured with Baf-A1 (the Baf-A1-treated group) and hepatoblastoma cells cultured in solvent DMSO in place of Baf-A1 (the Baf-A1-free group). In the Baf-A1-treated group, Baf-A1 was added to the culture at a final minimum concentration of 10 nM to observe the morphological changes in the hepatoblastoma cells.
Observations of morphological changes
Bafilomycin A1 was added to the hepatoblastoma cell culture media, and subsequent morphological changes were monitored over time by phase contrast microscopy. The hepatoblastoma cells were also observed by transmission electron microscopy at 24 h after the addition of Baf-A1. The observation was performed by the hat technique with Aclar films according to the method described by Bunge and Wood [7]. After 24 h of culture, the cells were prefixed with 0.1 M phosphate buffer (pH 7.4) containing 2.5% glutaraldehyde (for 1 h), post-fixed with 0.1 M phosphate buffer containing 1% osmium tetroxide (for 1 h), dried by ethanol, embedded in Epon, and sliced into ultrathin sections. The ultrathin sections were electron stained with uranyl acetate and citrate lead, then observed by a JEM-1010 80 kV transmission electron microscope (JEOL, Tokyo, Japan).
Apoptosis detection using flow cytometry
The cell apoptosis was detected with a flow cytometer using the Annexin V-FITC method [via the binding of phosphatidyl serine (PS) exposed to the cell membrane surface] and FITC-labeled Annexin with an Annexin V-FITC Apoptosis Detection Kit (BioVision, CA, USA). Binding buffer, FITC-labeled Annexin V and propidium iodide (PI) were added one by one to a cell-suspended liquid, and reacted. The data were analyzed by Cell Quest Pro (Version 5.1.1) after measurement with the flow cytometer FACScan (Becton Dickinson, BD, Tokyo, Japan). Annexin-V-positive, PI-negative cells were defined as early apoptotic cells, and Annexin V-positive, PI-positive cells were defined as late apoptotic cells.
Gene array analysis
Total RNA was extracted from cultured human hepatoblastoma cells (in the Baf-A1-treated group and Baf-A1-free group) by the TRIZOL (Invitrogen Corp., Carlsbad, CA, USA) method 22 h after adding reagent. Extracted RNA was further purified by an RNeasy MinEluteTM cleanup kit (Qiagen, Hilden, Germany). An Affymetrix GeneChip Expression Array, ‘custom order’ (Human Genome U133 Plus 2.0) by KURABO INDUSTRIES Ltd. (Osaka, Japan), was used for the human whole genome array analysis. The double strand cDNA was synthesized from purified 2 μg of total RNA from the cultured human hepatoblastoma cells (Baf-A1-treated group and Baf-A1-free group) using T7 oligo dT primers, and the biotin signal cRNA was synthesized by in vitro transcription. The biotin signal cRNA was hybridized into the array after adding hybridization fluorescent dye, and the image obtained was quantified after the cRNA was detected. Next, the array analysis data of the Baf-A1-treated group and the Baf-A1-free group were compared, alterations of gene expressions were detected and quantified, and the rates of expression of the Baf-A1-treated and Baf-A1-free groups were compared.
Differences between the expression level of a Baf-A1-treated group and Baf-A1-free group are shown with a Signal Log Ratio using a dual logarithm in each probe pair. In calculating Log 2 (signal of Baf-A1-treated group/signal of Baf-A1-free group), the average value was expressed as ‘1’ if the signal of the Baf-A1-treated group was expressed twice compared with signal of the Baf-A1-free group. Conversely, the average value was expressed as ‘−1’ if the signal of the Baf-A1-free group was expressed twice compared with the signal of Baf-A1-treated group. The obtained data were analyzed using the DNA MicroArray Viewer analysis system, and 27 V-ATPase related genes were extracted.
Effect of bafilomycin A1 on normal human hepatocytes
Culture and adjustment of normal human hepatocytes
Normal human hepatocyte cell lines purchased from ScienCell Research Laboratories (CA, USA) were cultivated in 5% CO2 at 37°C using a Hepatocyte Medium specified by the supplier, and used for the test upon reaching confluency.
Experimental groups
The experimental groups included normal hepatocytes cultured with Baf-A1 (the Baf-A1-treated group) and normal hepatocytes cultured in solvent DMSO in place of Baf-A1 (the Baf-A1-free group). In the Baf-A1-treated group, Baf-A1 was added into the liquid media to a final concentration of 10 nM added, as in the experiments with the hepatoblastoma cells.
Observations of morphological changes
Bafilomycin A1 was added to cultured normal human hepatocytes, and subsequent morphological change was monitored over time by phase contrast microscopy.
Results
Effect of bafilomycin A1 on human hepatoblastoma cells
Morphological changes
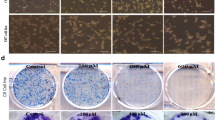
Morphological changes of human hepatoblastoma cells in Baf-A1-treated group were monitored over time by phase contrast microscopy (at 0, 6, 12, 24, 30, and 48 h after the addition of the reagent). At 24 h after addition of Baf-A1, the cancer cells were detached from the culture dishes and deformed, with substantial morphological changes. Significant reductions in the cell count and significant deformation were observed at 30 and 48 h after the addition of reagent (Fig. 1).
Sequential changes of hepatoblastoma cells after the addition of Baf-A1. Under observation with a phase contrast microscope, the hepatoblastoma cells broke away from the culture plate and changed shape 24 h after the addition of Baf-A1. A clear reduction in cell numbers and deformation was observed over time thereafter: 30 and 48 h after the addition of reagent
Hepatoblastoma cells were observed at 24 h after the addition of Baf-A1 by transmission electron microscopy. Apoptotic bodies and condensation of nuclear chromatin to the peripheral areas of the nuclear membrane were observed, verifying the characteristic findings of apoptosis (Fig. 2).
Apoptosis detection by flow cytometry
At 48 h after the addition of Baf-A1, the phosphatidyl serine (PS) expression was measured on the cell membrane surfaces of the Baf-A1-treated group and Baf-A1-free group by the Annexin V-FITC method, and apoptotic hepatoblastoma cells were detected. The Annexin-V-positive cells were significantly increased in the hepatoblastoma culture at 48 h after the addition of Baf-A1. Apoptotic hepatoblastoma cells were increased in the Baf-A1-treated cells than in the Baf-A1-free cells in both the early apoptosis cell group (PI-negative, Annexin-V-positive) and the later apoptosis cell group (PI-positive, Annexin-V-positive) (Fig. 3).
Detection of apoptosis in hepatoblastoma cells by Annexin-FITC. The Baf-A1-treated group showed a greater increase than the Baf-A1-free group in both early apoptotic cells (PI negative, Annexin V positive) and late apoptotic cells (PI positive, Annexin V positive) (48 h after the addition of Baf-A1)
Gene array analysis
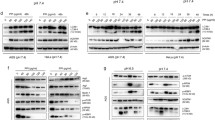
We performed a whole human genome array analysis using the GeneChip Expression Analysis on hepatoblastoma cells (the Baf-A1-treated group and Baf-A1-free group at 22 h after adding reagent). The results of the analysis of the V-ATPase-related gene expression showed that three genes out of 27 V-ATPase related transcripts (ATP6V0D2, ATP6V1B1, and ATP6V0A1) were more weakly expressed in the Baf-A1-treated group than in the Baf-A1-free group (Fig. 4; Table 1).
The effect of bafilomycin A1 on normal human hepatocytes
Observation of the morphological changes
The morphological changes of cultured normal human hepatocytes in the Baf-A1-treated group and Baf-A1-free group were observed by a phase contrast microscope at 0, 24, and 48 h after the reagent was added (Fig. 5). Minimal Baf-A1-induced inhibition of cell growth was observed in the normal human hepatocytes.
Discussion
Infant and child cases of primary liver carcinoma cannot be expected to completely recover without complete resection, and anticancer drugs are used to achieve the resection. The treatment strategy in Japan, however, differs from that in Europe, where all patients undergo preoperative chemotherapy. Japanese patients undergo initial primary surgery when complete resection is inevitable, and while chemotherapy takes priority in even so-called ‘resectable patients’, to raise the safety and rate of complete resection.
In the United States, the Children Oncology Group (COG) is conducting ongoing trials of cisplatin, vincristine, and fluorouracil for postoperative hepatoblastoma patients. Liver Tumor Strategy Group (SIOPEL) in Europe has proposed a staging system called PRETEXT based on the tumor site in the liver. They are also conducting studies to compare cisplatin alone with cisplatin + doxorubicin (PLADO), and to examine the validity of preoperational chemotherapy [8]. Japanese Study Group for Pediatric Liver Tumor (JPLT), formed in Japan in 1991, has been administering a chemotherapy with an original protocol. The overall 2-year probability of survival of patients receiving a preoperational chemotherapy of intravenous administration of two agents, cisplatin and THP-adriamycin, is comparable to the results from COG and SIOPEL, at 81%. With advanced cancers, however, the rate of complete resection is 55% or less, no better than the results from the West, and the patients with unresectable cancers never survive without tumors. In consideration of these factors, a salvage therapy of four agents, that is, carboplatin, ifosfamide, VP-16, and THP-adriamycin, has been used for tumors that do not become resectable by the JPLT-2 protocol. Even with this salvage therapy, the prognosis is very poor for patients with advanced cancers and patients with metastasis during treatment, and healing by the usual courses of chemotherapy is thought to be very difficult. The introduction of chemotherapy with newly developed drugs is indispensable.
Animal cells are composed of diverse cell organelles (acid organelles), including lysosome, endosome, synaptic vesicle, chromatin vesicle, and Golgi apparatus. The environments inside these organelles are kept within an acidic range (pH 4.5–6.5), creating conditions unlike those of cytoplasm [1]. Three types of H+-ATP enzyme play important roles in maintaining this acidic environment: P-ATPase, F-ATPase and V-ATPase. Of these three types, the V-ATPase enzymes are primarily involved in the acidification of the inside of the organelles. The V-ATPases take part in vesicular transport, protein processing, immune responses, etc. inside the organelle membranes. H+ is transported into the vacuoles via the hydrolysis energy of ATP, and a steady intravesical pH is maintained via the formation of H+ concentration gradient through the membranes [9].
The V-ATPases are also found in the plasma membranes of various cancer cells [10]. Whereas the extracellular pH around normal cells is neutral, that around cancer cells is acidic [11]. Compared with intracytoplasmic pH of normal cells, that of cancer cells shifts towards neutral or slight alkalinity [12]. In this slightly acidic environment, the plasma membrane V-ATPases of cancer cells facilitate the intracytoplasmic alkalization and extracellular acidification of cancer cells [13]. The acidification of the extracellular matrix of the cancer cells or the intercellular space by the V-ATPases of cancer cells is believed to play important roles in drug resistance, apoptosis resistance, and cancer invasion/metastasis. For this reason, inhibitors of V-ATPases are expected to serve as highly selective anti-cancer drugs [14, 15].
Macrolide antibiotics such as bafilomycin and concanamycin inhibit these functions of V-ATPases [16, 17] are attracting attention as therapeutic agents not only for cancer, but also for osteoporosis [18]. The osteoclast, a cell instrumental in bone reconstruction, adheres to the bone surface within the medullary cavity, forming bone resorption cavities. The inside of these bone resorption cavities (between the osteoclast cell membrane and the bone) is kept acidic through the ATPase and Cl− ion channels. The osteoclast-mediated bone resorption is thought to result from collagen degradations by the proteases and the dissolution of hydroxyapatite by acid in the bone resorption cavities. Therefore, the osteoclast-inhibiting activity of the V-ATPase inhibitors is also expected to confer a therapeutic effect for osteoporosis.
In this study, the effects of V-ATPase inhibitors on hepatoblastoma cell proliferation were observed morphologically with a phase microscope and a transmission electron microscope. The results showed more inhibition of cell growth in the Baf-A1-treated group than in the Baf-A1-free group in human hepatoblastoma cells, and the hepatoblastoma cells whose growth was inhibited showed morphological changes. In other words, hepatoblastoma cell growth was inhibited significantly by the specific V-ATPase inhibitor by Baf-A1. Moreover, our observations by electron microscopy confirmed apoptosis as the cause of this variation. The same finding has been confirmed by Ohta et al. [19] in pancreatic cancer cells and by Nakashima et al. [20] in gastric cancer cells. We also confirmed it in our own quantitative evaluation of apoptosis using flow cytometry (a significant increase of apoptotic cells was observed in the Baf-A1-treated hepatoblastoma cell group).
The plasma membrane V-ATPases of cancer cells are now believed to affect the level of malignancy and invasiveness of a cancer. Sennoune et al. [21] found plasma membrane V-ATPase in human breast cancer cells using immunocytochemistry and reported strong expression of V-ATPases in breast cancer cells in particular. They also found that the bafilomycin-sensitive ATP hydrolysis system is significantly more prevalent in metastatic breast cancer cells than in non-metastatic ones, and the plasma membrane V-ATPase expression is an essential factor in determining the level of invasiveness of a cancer cell and the acquisition of metastatic properties [22]. Ohta et al. [2, 19] found ATP6L, a subunit of V-ATPase, in pancreatic cancers, and concluded that it was expressed in invasive pancreatic cancers and not in non-invasive ones. We did not observe the expression of ATP6L, a subunit of V-ATPase, in our own RT-PCR experiment with hepatoblastoma cells. Data are not shown. It may be that the hepatoblastoma cells used in our experiment were a well-differentiated type with a relatively good prognosis.
V-ATPase, on the other hand, has been reported to include both the V1 domain (which contains the activation center for the ATPase hydrolysis reaction) and intramembrane V0 domain (which forms the proton channel), and both domains are reported to include several types of subunit and even isoforms [22, 23]. One study examined how V-ATPase genes and isoforms expressed tissue-specifically relate to diseases (tubular acidosis, marble bone disease, hearing loss, etc.), but no relationship between the ATPase gene and cancer cell proliferation was found.
Bafilomycin, a V-ATPase inhibitor, is thought to bind to the V0 domain of the V-ATPase [24]. Bowman et al. [25] reported that bafilomycin inhibits V-ATPase by binding to the subunit c in the V0 domain of V-ATPase and impeding the rotation of the c-subunit ring. Another report indicated that bafilomycin depends on subunit a of the V0 domain for the binding of the inhibitor [26]. In a cDNA array analysis by Otero-Rey et al. [27] to study the expression of V-ATPase in oral squamous cell carcinoma, the expression of ATP6V1C1 in the V1 domain was the strongest in oral squamous cell carcinoma. Lu X et al. [28], meanwhile, reported that ATP6V0C knockdown from the V0 domain using RNA-interference inhibited the V-ATPase activity in pancreatic cancer. ATPase genes may have cell-specific properties for different kinds of tissues and cancers, but the differences are still obscure.
According to results of the whole genome array by our GeneChip Expression Analysis on hepatoblastoma cells (the Baf-A1-treated group and Baf-A1-free group), three genes out of the 27 V-ATPase-related transcripts (ATP6V0D2, ATP6V1B1, and ATP6V0A1) were expressed significantly more weakly in the Baf-A1-treated group than in the Baf-A1-free group. Of the three genes that manifested down-regulation after the addition Baf-A1, two that code the isoforms in the intramembrane V0 domain and form the proton channel (i.e., ATP6V0D2 and ATP6V0A1) are thought to be playing a direct role in suppressing hepatoblastoma cell growth. In electron microscopy observations by Hinoki et al. [29], the lysosomes serving as ATPase localization sites swelled when Baf-A1 was added to the macrophages. V-ATPase has been observed in the plasma membrane of macrophages and cell organelles. In our study with hepatoblastoma cells, Baf-A1 may also act on the organelles from the plasma membranes.
On the other hand, little inhibition of cell growth was seen when Baf-A1 was added to the culture solution in the experimental group of normal human hepatocytes. Baf-A1 significantly suppressed hepatoblastoma cell growth, whereas it had little effect on normal human hepatocytes. The following reasons may explain this difference: V-ATPase is more strongly expressed in hepatoblastoma cells than in normal hepatocytes; the lysosomes, which become the target of the V-ATPase inhibitor, are not well-developed in normal cells compared to cancer cells; and the cell growth factor requirements differ. The differentiation-inducing and apoptosis-inducing properties of V-ATPase inhibitors are effective in suppressing hepatoblastoma cell growth. This suggests that V-ATPase inhibitors such as Baf-A1 may hold promise as therapeutic drugs targeting the hepatoblastoma cell V-ATPase.
Conclusion
This study proved that the V-ATPase inhibitor Baf-A1 selectively inhibits the growth of hepatoblastoma cells and HuH-6 clone-5, and induces apoptosis without significantly harming normal hepatocytes. This suggests that the V-ATPase inhibitors may hold promise as agents for the anti-cancer treatment of hepatoblastomas. As the details of the diverse and intricate control mechanisms of V-ATPase are revealed further, new drugs with hepatoblastoma cells as molecular targets may be developed in the future.
References
Nelson N et al (1989) Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr 21:553–571
Ohta T, Numata M, Yagishita H et al (1996) Expression of 16 kDa proteolipid of vacuolar-type H+-ATPase in human pancreatic cancer. Br J Cancer 73:1511–1517
Ohkuma S, Shimizu S, Noto M et al (1993) Inhibition of cell growth by bafilomycin A1, a selective inhibitor of vacuolar H+-ATPase. In vitro Cell Dev Biol Anim 29A:862–866
Manabe T, Yoshimori T, Henomatsu N et al (1993) Inhibitors of vacuolar-type H+-ATPase suppresses proliferation of cultured cells. J Cell Physiol 157:445–452
Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and perturbation of pH by various agents. Proc Natl Acad Sci USA 75:3327–3331
Nishihara T, Akifusa S, Koseki T et al (1995) Specific inhibitors of vacuolar type H+-ATPase induce apoptotic cell death. Biochem Biophys Res Commun 212:255–262
Bunge RP, Wood P (1973) Studies on the transplantation of spinal cord tissue in the rat. I. The development of a culture system for hemisections of embryonic spinal cord. Brain Res 57:261–276
Perilongo G, Shafford E, Plaschkes J (2000) SIOPEL trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol 1:94–100
Hurtado-Lorenzo A, Skinner M, Annan JEI et al (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Martinez-Zaguilan R, Lynch RM, Martinez GM et al (1993) Vacuolar-type H+-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol 265:1015–1029
Finbow ME, Harrison MA (1997) The vacuolar H+-ATPase: a universal proton pump of eukaryotes. Biochem J 324:697–712
Shrode LD, Tapper H, Gristein S (1997) Role of intracellular pH in proliferation, transformation, and apoptosis. Bioenerg Biomembr 29:393–399
Hinton A, Bond S, Forgac M (2007) V-ATPase functions in normal and disease processes. Pflugers Arch. doi: 10.1007/s00424-007-0382-4
Nelson N, Harvey WR et al (1999) Vacuolar and plasma membrane proton adenosinetriphoshatases. Physiol Rev 79:361–385
Yoshimoto Y, Imoto M (2002) Induction of EGF-dependent apoptosis by vacuolar type H+-ATPase inhibitors in A431 cells overexpressing the EGF receptor. Exp Cell Res 279:118–127
Hamada H, Moriyama Y, Maeda N et al (1990) Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun 170:873–878
Huss M, Sasse F, Kunze B, Kunze B et al (2005) Archazolid and apicularen: novel specific V-ATPase inhibitors. BMC Biochem 6:13
Niikura K, Takeshita M, Takano M et al (2005) A vacuolar ATPase inhibitor, FR 167356, prevents bone resorption in ovariectomized rats with high potency and specificity: potential for clinical application. J Bone Miner Res 20:1579–1588
Ohta T, Arakawa H, Futagami F et al (1996) A new strategy for the therapy of pancreatic cancer by proton pump inhibitor. Jpn J Cancer Chemother 23:1660–1664
Nakashima S, Hiraku Y, Oikawa ST (2003) Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J Biochem 134:359–364
Sennoune SR, Bakunts K, Martinez GM et al (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:1443–1452
Nishi T, Forgac M (2002) The vacuolar H+-ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3:94–103
Stevens TH, Forgac M (1997) Structure, function and regulation of the vacuolar H+-ATPase. Annu Rev Cell Dev Biol 13:779–808
Pali T, Whyteside G, Dixon N et al (2004) Interaction of inhibitors of the vacuolar H+-ATPase with the transmembrane Vo-sector. Biochemistry 43:12297–12305
Bowman EJ, Bowman BJ (2005) V-ATPases as drug targets. J Bioenerg Biomembr 37:431–435
Whyteside G, Meek PJ, Ball SK et al (2005) Concanamycin and indolyl pentadieneamide inhibitors of the vacuolar H+-ATPase bind with high affinity to the purified proteolipid subunit of the membrane domain. Biochemistry 44:15024–15031
Otero-Rey EM, Somoza-Martin M, Barros-Angueira F et al (2008) Intercellular pH regulation in oral squamous cell carcinoma is mediated by increased V-ATPase activity via over expression of the ATP6V1C1 gene. Oral Oncol 44:193–199
Lu X, Qin W, Li J et al (2005) The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 65:6843–6849
Hinoki A, Yoshimura K, Fujita K et al (2006) Suppression of proinflammatory cytokine production in macrophages by lansoprazol. Pediatr Surg Int 22:915–923
Acknowledgments
We thank Ms. Kumiko Komatsu and Ms. Sachiko Matsumoto from the Division of Morphological Science at Saitama Medical University for their collaborations in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morimura, T., Fujita, K., Akita, M. et al. The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr Surg Int 24, 1087–1094 (2008). https://doi.org/10.1007/s00383-008-2229-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2229-2