Abstract
Patch closure is necessary to achieve tension-free repair in large congenital diaphragmatic hernia. However, the use of prosthetic material may lead to granulation, allergic reaction, infection, recurrence of hernia, and thoracic deformity. Tissue engineering may become an alternative treatment strategy for diaphragmatic hernia repair, since the regenerated autologous tissue is expected to grow potentially without rejection or infection. We evaluated the efficacy of diaphragmatic hernia repair in a rat model using a poly-lactic-co-glycolic acid (PLGA) mesh–collagen sponge hybrid scaffold, designed for in situ tissue engineering. Twenty-four F344 female rats were used. Oval-shaped defects were surgically created in the left diaphragm and repaired with three different grafts, including PLGA mesh in group 1 (n = 7), PLGA mesh–collagen sponge hybrid scaffold in group 2 (n = 7), and PLGA mesh–collagen sponge hybrid scaffold seeded with bone marrow-derived mesenchymal stem cells (MSCs) in group 3 (n = 10). The animals were killed at 1, 2, and 3 months after operation. The specimens were examined macroscopically and microscopically. No recurrence or eventration was observed. In all animals, autologous fibrous tissue with vascularization was generated at the graft site. Although no muscular tissue was detected, scattered desmin-positive cells were observed in groups 2 and 3. The ‘neodiaphragm’ in groups 2 and 3 was significantly thicker compared with that in group 1. There was no significant difference in the ‘neodiaphragm’ between groups 2 and 3. The PLGA mesh–collagen sponge hybrid scaffold provided better promotion of autologous in situ tissue regeneration in the diaphragm, suggesting its potential application to diaphragmatic repair in place of other prosthetic patches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthetic materials, including polypropylene mesh, polytetrafluoroethylene (PTFE) patch, reinforced silastic sheet, and polyethylene mesh, have been used for repair of large congenital diaphragmatic hernias (CDH) [1–8]. Allogenic lyophylized dura or autologous tissues such as fascia or muscle flaps have also been utilized [9–12]. Such prosthetic materials may possibly cause granulation, allergic reaction, and infection. They do not grow with the infant, and their use may furthermore result in hernia recurrence or thoracic deformity due to restricted chest wall development [13]. Although the use of autologous grafts such as muscle flaps to repair a large defect might minimize the possibility of such complications, these techniques are rather complicated and invasive, and require prolonged operating times. These may lead to massive bleeding when ECMO is applied in association with CDH repair. Recently, tissue engineering has become an alternative treatment to reconstruct such defects in humans [14–16]. It may be possible to replace the defect of the diaphragm by regenerated autologus tissues, which will also grow with the infant [16]. The aim of this study was to evaluate diaphragmatic repair using a novel scaffold designed for tissue engineering, PLGA mesh–collagen sponge hybrid scaffold, in a rat model [17, 18]. Furthermore, we investigated whether supplying some type of cells such as bone marrow-derived mesenchymal stem cells (MSCs) that can differentiate into muscle cells could contribute to better regeneration of the diaphragm.
Materials and methods
PLGA mesh–collagen sponge hybrid scaffold fabrication
The hybrid scaffold was prepared by forming collagen microsponges in the openings of a PLGA-knitted mesh as previously described [17]. Briefly, a Vicryl-knitted mesh made of polylactin 910 (a 90:10 copolymer of glycolic acid and lactic acid, PLGA, Ethicon, Inc., Somerville, NJ) was immersed in a bovine collagen acidic solution (type I, pH 3.0, 0.5 wt%, Koken Co., Ltd, Tokyo, Japan), and frozen at −80°C for 12 h. It was then freeze-dried under a vacuum of 0.2 Torr for 24 h to allow the formation of collagen microsponges. The collagen microsponges were further crosslinked by treatment with glutaraldehyde vapor from a saturated 25% glutaraldehyde aqueous solution at 37°C for 4 h. After crosslinking, the sponge was treated with a 0.1 M glycine aqueous solution to block unreacted aldehyde groups. After being washed with deionized water and freeze-dried, the scaffold was ready for use by sterilization with ethylene oxide.
Isolation and culture of bone marrow-derived mesenchymal stem cells
Isolation and primary culture of MSCs from the donor rats was performed according to the previously described method [19]. Female F344 rat femoral bones were collected and both ends of the long bones were cut away from the diaphyses. The bone marrow plugs were hydrostatically expelled from the bones using complete medium, consisting of Dulbecco’s modified Eagle’s medium (DMEM) containing selected lots of 10% calf serum and 1% penicillin/streptomycin (Gibco Laboratories, Boston, MA) in a humidified atmosphere of 5% CO2. The marrow plugs were disaggregated and the dispersed cells were centrifuged and resuspended twice in complete medium. Each primary culture was passaged twice to three new plates, and the cell density of the colonies was grown to approximately 90% confluence.
Experimental design
Twenty-four female F344 rats of inbred strain (12–31 weeks of age; weight 150–200 g) were used as recipients. The rats were divided into three groups: group 1 in seven rats, two layers of the PLGA mesh were used to replace the diaphragmatic defect; group 2 seven rats received two layers of the PLGA mesh–collagen sponge hybrid scaffold; group 3 eight rats were repaired with two layers of the PLGA mesh–collagen sponge hybrid scaffold seeded with approximately 1–2 × 106 MCS in-between.
Surgical procedures
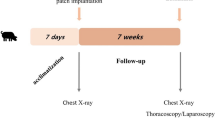
The rats were anesthetized with inhalation of ether and intramuscular ketamine injection, and then intubated using an external cylinder of 14G indwelling needle and put on a respirator. Laparotomy was performed through subcostal incision. An oval-shaped defect (1.5 × 1 cm2) was created in the left diaphragm. Then the defect was repaired with the group-specific scaffold (2.0 × 1.5 cm2) with a 4-0 running nonabsorbable suture. The abdominal side of the patch graft was covered with the greater omentum. Animals were allowed food and water shortly after completion of the procedure.
Evaluation
Two rats in each group were sacrificed at 1 and 2 months after the operation. Three rats in group 1, three rats in group 2, and six rats in group 3 were evaluated at three months. The trunk of the animal was transected immediately above and below the left diaphragm. The left hemidiaphragm was assessed to determine if the patch was intact without evidence of eventration or herniation. The left diaphragm with the patch graft was removed en block. The paraffin-embedded sections were stained with H&E and Masson’s Trichrome (MT). Immunohistochemical study was performed using mouse antidesmin monoclonal antibodies (1:100, DAKO, Kyoto, Japan). The DAKO Envision + detection system using dextran polymers conjugated with horseradish peroxidase (DAKO, Kyoto, Japan) was employed for detection. The nuclei were counterstained with hematoxyline.
Statistical analysis
The thickness of the regenerated tissues in groups 1, 2, and 3 was measured in the central and marginal portions of the patch, and the mean thickness at 3 months after surgery was compared using Student’s t test. Values of P < 0.05 were considered statistically significant.
These animal experiments were consistent with the University of Tsukuba’s Regulation of Animal Experiment and permitted by the Animal Experiment Committee, University of Tsukuba.
Results
Macroscopic examination
No animal showed mechanical breakdown or diaphragmatic eventration. There was no marked difference of the surface of the ‘neodiaphragm’ among all three groups. Gross examination of the patches at the time of death revealed an intact suture line. Moderate adhesions were observed between the patches and the superior surface of the left lobe of the liver or the greater omentum interposed at surgery. Neovascularization on the surface of the graft site was observed. The mean maximum diameters of the graft at 1, 2, and 3 months after the operation were 1.8, 1.5, 1.7 cm in group 1, 1.5, 1.7, and 1.7 cm in group 2, and 1.7, 1.8, and 1.7 cm in group 3, respectively.
Microscopic examination
All patch grafts examined were viable without evidence of necrosis and maintained structural integrity without eventration. In each group, membranous linings of fibrous tissues over the scaffolds and inflammatory cell infiltration into and around the scaffold were observed at 1 month. The inflammatory findings disappeared after 2 months. PLGA mesh was partly absorbed with blurring of the original structure within a month, and the basic structures of PLGA mesh had almost disappeared in 2 or 3 months. Ingrowth of fibroblasts into the graft was observed in all groups. The mean thickness around the center of the ‘neodiaphragm’ was 200 μm in group 1, and 550 μm in groups 2 and 3. The mean values of the marginal thickness of the ‘neodiaphragm’ were 600 μm in group 1, 1,000 μm in group 2, and 1,250 μm in group 3. The regenerated tissues in groups 2 and 3 were significantly thicker than that in group 1 in the central as well as marginal portions (P < 0.05). Although regeneration of muscular structure could not be observed in any of the three groups even 3 months after operation, scattered desmin-positive cells were found within the graft in groups 2 and 3 (Figs. 1, 2). There was little difference between groups 2 and 3 in microscopic findings, indicating no benefit of MSC seeding in the scaffold.
Discussion
Prosthetic patches have been routinely used to close large diaphragmatic defects in neonates and infants. Implantation of these synthetic materials exposes patients to the risks of not only rejection, infection and recurrence due to patch displacement or repair disruption, but also chest wall deformity in long-time survivors. Recent advances in the perinatal care of infants with severe CDH have dramatically lowered mortality rates. Namely, the number of survivors with severe CDH who usually require a prosthetic patch for tension-free closure of larger defects has been increasing. This in turn has led to an increase in recurrence rates following repair [10–13]. We hypothesized that, to lower the risk of morbidity and recurrence, an autologous, tissue-engineered patch might be of benefit.
Poly-lactic-co-glycolic acid (PLGA) (Vicryl) is a three-dimensional carrier that is bioabsorbable, durable and easy to process, and has been used clinically in various surgical procedures. Synthetic polymers such as PLGA possess good mechanical properties, but are relatively hydrophobic and hinder cell seedings. In contrast, naturally derived polymers, such as collagen, offer hydrophilic cell adhesiveness as well as cell-proliferative activity, but have poor mechanical strength. Therefore, the PGLA mesh–collagen sponge hybrid scaffold has been designed to combine their advantages. We have previously shown the usefulness of the hybrid scaffold, prepared by combining synthetic polymer for mechanical strength with naturally derived collagen for cell seeding [17, 18]. Previous studies have shown that the use of PLGA mesh for diaphragmatic repair in the growing rat resulted in a high incidence of eventration because of insufficient fibrous tissue [20]. Subsequently, collagen-coated PLGA mesh was used to reinforce the resutured muscle flap repair as well as the primary repair in clinical patients with agenesis of the left diaphragm, but the risk of eventration was reported [5]. These results might be due to the insufficient proliferation of fibroblasts caused by the constant stress induced by diaphragmatic motion. In this study, we combined collagen sponge, which is expected to promote cell proliferation in situ more efficiently than collagen coating. We also compared the results after the repair between this hybrid scaffold with and without MSC seeding. Plain PLGA mesh sheets were used as a control. We sequentially assessed the results at 1, 2, and 3 months after the repair, because this hybrid scaffold was designed to be absorbed by 3 months. The promotion of in situ tissue regeneration by use of this hybrid scaffold led to the formation of a much thicker autologous tissue with capillary incorporation in the defect of the diaphragm than that observed when plain PLGA mesh was utilized. Although obvious muscle regeneration was not observed, scattered desmin-positive cells were observed in the regenerative tissues in groups 2 and 3. Probably, these desmin-positive cells would be of host origin, because they were found without MSC seeding. The seeding of MSC on the hybrid scaffold did not bring about any advantage in the present histological study, although functional evaluations were not performed. Although further validation of its usefulness in CDH repair in growing animals may be required, our study suggested that PLGA mesh–collagen sponge hybrid scaffold could possibly represent a useful material by providing autologous in situ tissue regeneration and growth potential.
References
Dalton ML Jr, Dixon RB, West RL (1966) Dacron diaphragmatic grafts. Am J Surg 111:668–672. doi:10.1016/0002-9610(66)90038-9
Geisler F, Gottleib A, Fried A (1977) Agenesis of the right diaphragm repaired with marlex. J Pediatr Surg 12:587–588. doi:10.1016/0022-3468(77)90204-4
Touloukian RJ (1978) A “new” diaphragm following prosthetic repair of experimental hemidiaphragmatic defects in the pup. Ann Surg 187:47–51. doi:10.1097/00000658-197801000-00009
Newman BM, Jewett TC, Lewis A, Cerny F, Khan A, Karp M et al (1985) Prosthetic materials and muscle flaps in the repair of extensive diaphragmatic defects: an experimental study. J Pediatr Surg 20:362–367. doi:10.1016/S0022-3468(85)80220-7
Lally KP, Cheu HW, Vazquez WD (1993) Prosthetic diaphragm reconstruction in the growing animal. J Pediatr Surg 28:45–47. doi:10.1016/S0022-3468(05)80352-5
Clark RH, Hardin WD Jr, Hirschl RB, Jaksic T, Lally KP, Langham MR Jr et al (1998) Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg 33:1004–1009. doi:10.1016/S0022-3468(98)90522-X
Kimber CP, Dunkley MP, Haddock G, Robertson L, Carey FA, Cuschieri A (2000) Patch incorporation in diaphragmatic hernia. J Pediatr Surg 35:120–123
Saltzman DA, Ennis JS, Mehall JR, Jackson RJ, Smith SD, Wagner CW (2001) Recurrent congenital diaphragmatic hernia: a novel repair. J Pediatr Surg 36:1768–1769. doi:10.1053/jpsu.2001.28818
Koot VCM, Bergmeijer JG, Molenaar JC (1993) Lyophylized dura patch repair of congenital diaphragmatic hernia: occurrences of relapses. J Pediatr Surg 28:667–668. doi:10.1016/0022-3468(93)90027-I
Sydorak RM, Hoffman W, Lee H, Yingling CD, Longaker M, Chang J et al (2003) Reversed latissimus dorsi muscle flap for repair of recurrent congenital diaphragmatic hernia. J Pediatr Surg 38:296–300. doi:10.1053/jpsu.2003.50097
Scaife ER, Johnson DG, Meyers RL, Johnson SM, Matlak ME (2003) The split abdominal wall muscle flap—a simple, mesh-free approach to repair large diaphragmatic hernia. J Pediatr Surg 38:1748–1751. doi:10.1016/j.jpedsurg.2003.08.045
Rosenkrantz JG, Cotton EK (1964) Replacement of left hemidiaphragm by a pedicled abdominal muscular flap. J Thorac Cardiovasc Surg 48:912–920
Moss RL, Chen CM, Harrison MR (1992) Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J Pediatr Surg 27:754–756. doi:10.1016/S0022-3468(05)80109-5
Fauza DO, Marler JJ, Koka R, Forse RA, Mayer JE, Vacanti JP (2001) Fetal tissue engineering: diaphragmatic replacement. J Pediatr Surg 36:146–151. doi:10.1053/jpsu.2001.20034
Dalla Vecchia L, Engum S, Kogon B, Jensen E, Davis M, Grosfeld J (1999) Evaluation of small intestine submucosa and acellular dermis as diaphragmatic prostheses. J Pediatr Surg 34:167–171. doi:10.1016/S0022-3468(99)90250-6
Sandoval JA, Lou D, Engum SA, Fisher LM, Bouchard CM, Davis MM et al (2006) The whole truth: comparative analysis of diaphragmatic hernia repair using 4-ply vs 8-ply small intestinal submucosa in a growing animal model. J Pediatr Surg 41:518–523. doi:10.1016/j.jpedsurg.2005.11.068
Chen G, Ushida T, Tateishi T (2000) A hybrid network of synthetic polymer mesh and collagen sponge. Chem Commun (Camb) 16:1505–1506. doi:10.1039/b003508o
Tateishi T, Chen G, Ushida T (2002) Biodegradable porous scaffolds for tissue engineering. J Artif Organs 5:77–83. doi:10.1007/s100470200014
Lennon DP, Caplan AI (2006) Isolation of rat marrow-derived mesenchymal stem cells. Exp Hematol 34:1606–1607. doi:10.1016/j.exphem.2006.07.015
Ramadwar RH, Carachi R, Young DG (1997) Collagen-coated Vicryl mesh is not a suitable material for repair of diaphragmatic defects. J Pediatr Surg 32:1708–1710. doi:10.1016/S0022-3468(97)90510-8
Acknowledgments
This work was supported by Grant-in-Aids from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urita, Y., Komuro, H., Chen, G. et al. Evaluation of diaphragmatic hernia repair using PLGA mesh–collagen sponge hybrid scaffold: an experimental study in a rat model. Pediatr Surg Int 24, 1041–1045 (2008). https://doi.org/10.1007/s00383-008-2212-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-008-2212-y