Abstract
Recent studies of testicular descent suggest not only that the gubernaculum does not initially attach to the scrotum, but also that it must migrate from the groin. Two findings suggest that the gubernaculum may behave like an embryonic limb bud during this phase. First, the active growth centre is at the distal tip of the gubernaculum. Secondly, the gubernaculum is loose in the subcutaneous tissues beneath Scarpa's fascia. The free protrusion of the gubernaculum from the abdominal wall was so reminiscent of a developing embryonic limb bud, we thought that the biological controls of both processus may be similar. This review examines what is known about vertebrate limb bud development, and compares the mechanisms to what has recently been discovered in the gubernaculum. The hypothesis that both processes may be similar is initially consistent with the current facts, encouraging us to investigate this further experimentally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, Hrabrovsky et al. [1] identified the gubernacular tip as the major area of active cell proliferation, analogous to the “progress zone” of the developing limb bud. In this review we compare the mechanisms of limb bud initiation, outgrowth and patterning with the process of testicular descent involving the gubernaculum, as a source of potential new studies in the latter area.
Overview of limb bud formation
The vertebrate limb consists of a proximal stylopod (humerus;femur), a zeugopod (ulna/radius; fibula/tibia), and a distal autopod (carpals, metacarpals, phalanges; tarsals, metatarsals, phalanges). The limb develops from a limb bud, which forms at a specific position along the body axis. It develops according to a specific pattern with arrangement in three dimensions: the proximal–distal axis (shoulder to finger-tips), anterior–posterior axis (thumb to little finger), and dorsal–ventral axis (knuckles to palm). The proximal–distal axis is broadly regulated by members of the fibroblast growth factor (FGF) family, in conjunction with patterning information from Hox genes. The anterior–posterior axis is mainly regulated by Sonic Hedgehog (SHH), and signalling molecules in the ectoderm predominantly regulate the dorsal–ventral axis. However, these regulatory factors interact extensively to determine overall organisation of the limb [2].
Limb bud initiation
Limbs form at discrete positions along the body axis called limb fields, where their position is constant with respect to the level of HOX gene expression along the rostral–caudal axis. The limb field is described as a ‘harmonious equipotential system’ where any part of this field, even when segmented, is able to form a full limb. It appears that retinoic acid signalling in the lateral plate mesoderm is required to make the tissue competent to form limb bud fields [3]. The limb bud consists of a core of mesenchymal cells surrounded by a layer of ectoderm. The two groups of cells in the mesenchyme are the cells from the limb field lateral plate mesoderm, which become the limb skeletal precursors, and cells from the somites, which migrate into the limb fields and give rise to the limb muscle precursors. The ectoderm gives rise to the epidermis of the limb. Specification of the limb buds as either a forelimb or hindlimb was thought to be determined by T-box transcription factors—Tbx5 in forelimb specification and Tbx4 in hindlimb specification [4]. However, further work has shown that limb specification occurs earlier than Tbx gene expression and, at least in the hindlimb, additional factors are involved [5].
Proximal–distal axis
Two models have been proposed to describe the specification of the proximal–distal axis of the limb: the progress zone model (PZ) and the early specification model. The progress zone model (reviewed in 6) [6] proposes that each mesoderm cell is specified by the amount of time it spends dividing in the progress zone. Cells exit the progress zone constantly as the limb grows. When cells exit the PZ they become fixed with the positional information last gained in the PZ. This means cells leaving first form the stylopod and cells that leave last become the autopod. The early specification model [7] postulates that specification of limb segments occurs at a very early stage of development. There are distinct domains within the limb bud, which expand as required prior to differentiation. In both models there is an area of undifferentiated cells towards the distal end of the limb bud (Fig. 1).
Schematic diagrams of limb bud outgrowth in longitudinal sections. The apical ectodermal ridge (AER) covers the underlying mesenchyme, which contains an undifferentiated growth centre or “progress zone” (PZ). Proximo-distal development is proposed to occur by either a the progress zone model, where cells exit the PZ and differentiate into stylopod, zeugopod or autopod, depending on time spent in the PZ. Alternatively, the early specification model (ES), shown in b suggests that all regions of the limb are already preprogrammed in the progress zone
Hox genes play a role in specifying the limb region identity, where different genes specify the stylopod, zeugopod or autopod. The Hoxa and Hoxd clusters have been most extensively studied and each gene has been shown to have a unique expression domain in the developing limb [8].
During the specification of the stylopod there is nonpolar expression of Hoxd-9, Hoxd-10, Hoxa-9 and Hoxa-10. During specification of the zeugopod there is sequential activation of Hoxd-9 through Hoxd-13, which are posteriorly polarized and uniform expression of Hoxa-11. During specification of the autopod, Hoxa-13 expression is activated at the posterior border of the limb bud followed in order by Hoxd-13, Hoxd-12, Hoxd-11 and Hoxd-10. While the time of Hox gene expression is known it is still unclear when they affect patterning.
The source of signals regulating proximodistal patterning is the Apical Ectodermal Ridge (AER), a specialised ectodermal structure that runs along the distal tip of the limb bud. Expression of Fgf10 in the mesenchyme of the limb bud is required for the induction of the AER (Fig. 2) [9]. Fgf10 acts through inducing and maintaining the expression of Fgf8 in the AER [10]. The AER expresses FGF’s (Fgf8, as well as Fgf4, and to a lesser extent Fgf9 and Fgf17) [11], which act to promote the survival of the underlying mesenchyme, probably by preventing apoptosis [12]. Fgf8 also maintains expression of Fgf10 in a positive feedback loop, allowing the continued outgrowth of limbs.
Anterior–posterior axis
The anterior–posterior limb axis is specified by the zone of polarizing activity (ZPA), a small block of mesodermal tissue near the posterior junction of the limb bud and body wall. The key regulators associated with the ZPA are retinoic acid (RA), Sonic Hedgehog (SHH) and bone morphogenic proteins (BMP’s). Shh and Bmp’s, in particular Bmp2, are expressed in the ZPA, and the ectopic application of RA or Shh to an anterior limb region results in digit duplications as occur with ZPA transplants [13].
Retinoic acid is important in the establishment of the ZPA [14] and it is now recognised that this occurs through factors that control the response of cells to retinoic acid signalling. It is thought that RA probably patterns the proximal limb segment while Shh and Bmp’s cooperate to pattern the middle and distal segments. It is likely that a process of sequential signalling occurs whereby Shh controls the width of the limb and digit number, and Bmp’s act on cells primed by Shh to determine digit type [15].
Hox genes are also involved in the regulation of the AP axis in two different ways [16]. In the early limb bud there is posterior restriction of Hoxd gene products, which sets up the anterior–posterior prepatterning and determines the local activation of Shh. Later Shh controls the transcription of Hoxd genes involved in generating distal limb structures.
Two other transcription factors play key roles in the AP organization of the limb. Throughout limb bud growth Gli3 is anteriorly restricted, while dHand is posteriorly restricted. It is thought that these interact with each other, as well as Shh and Hox genes, but the mechanisms are not fully understood [17].
Dorsal–ventral axis
Known regulators of dorsal–ventral limb patterning include three factors: Wnt7a expressed in the dorsal ectoderm; En-1 expressed in the ventral ectoderm and Lmx1b expressed in the dorsal mesoderm. Studies have shown that En-1 suppresses the expression of Wnt7a in the ventral ectoderm and Wnt7a induces the expression of Lmx1b in the dorsal mesoderm [18]. However, all three appear to have additional roles in the patterning of other limb axes.
Coordination of the three axes
The three axes of limb development and all interrelated and coordinated through processes that are not yet fully understood. Regulatory factors that define one axis are often used to maintain and define another axis.
The AER is induced by a mesenchymal signal, Fgf10, but forms at the interface between the dorsal and ventral ectoderm. This implies that there is likely to be a relationship, and the position of the AER is thought to be influenced by an ectodermal signal. An early link between the D–V axis and the AER is BMP signalling from the ventral limb ectoderm. BMP controls D–V patterning by acting upstream of En-1 to promote ventral patterning. BMP signalling also leads to the induction of the AER in an independent manner. This latter pathway appears to be indirect, occurring through downstream effectors (MSX proteins) [19]. However later it appears that En-1, and not BMP, is required for the maintenance of the AER.
The AER and the ZPA mutually support each other through a positive feedback loop of Shh and FGF’s. FGF signals from the AER induce (within the region of competence) and maintain Shh expression in the ZPA. In turn, Shh maintains the expression of several Fgf’s in the AER, including Fgf4, Fgf9 and Fgf17. With Fgf4, and maybe the others, this occurs through up-regulation of Gremlin, a BMP antagonist that prevents Bmp’s from down-regulating Fgf4.
Wnt7a from the dorsal ectoderm is also required to maintain normal expression of Shh from the ZPA [20].
These interactions provide a simple framework linking the signalling centres controlling each axis of growth, but undoubtedly the actual process is much more complex.
Overview of testicular descent
The urogenital ridge develops from the intermediate mesoderm and will develop into the gonads, kidney and genital ducts. The gubernaculum forms from lateral plate mesoderm, and attaches the testis and epididymis by an undifferentiated column of mesenchyme to the developing abdominal wall muscles. It forms by the sixth week of gestation in humans, and plays an important role in testicular descent. Testicular descent in a human can be divided into two main stages, with a putative period between to allow androgenic masculinization of the nervous system.
Trans-abdominal descent
The testis develops in the urogenital ridge as the mesonephros regresses, in an analagous position to the developing ovary in a female. As with the ovary, the testis undergoes relative descent within the abdomen as the lumbar vertebral region grows. However, unlike the ovary, the testis continues to descend to near the deep ring of the future inguinal canal between the 10th and 15th week of gestation, where it remains as the abdomen lengthens. Human abdominal wall musculature is developed by the sixth week with a gap, filled with gubernacular mesenchyme, through which the genitofemoral nerve (GFN) runs. This is the site of the future inguinal ring.
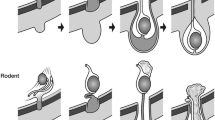
The gubernaculum, described by Hunter as the testiculo-inguinal ligament, is the cord of mesenchyme attaching the developing testis and epididymis to the inguinal abdominal wall. It precedes the testis by enlarging through the future inguinal ring of the anterior abdominal wall muscles (Fig. 3).
Schema showing the parts of the gubernaculum formed by the peritoneal diverticulum (processus vaginalis) developing within it. Plica gubernaculi: the central cord attached to epididymis and testis; Pars vaginalis gubernaculae: the outer rim where cremaster muscle develops; pars infravaginalis gubernaculae: the distal tip of the gubernaculum that swells into a bulbous end (the “bulb”) during the first phase of descent
The genitofemoral nerve (GFN) arises from L1 and L2 of the spinal cord. It has both motor and sensory branches and exits onto the anterior surface of the psoas muscle, running dorsally to the ureter. It crosses over the iliac vessels to reach the inguinal canal and supplies minor branches along the way to the prepucial gland. At this point the GFN bifurcates into genital and femoral branches, with the latter supplying sensation to the skin over the femoral triangle. The genital branch supplies motor fibres to the cremaster muscle, and sensation to the skin of the scrotum and the medial aspect of the thigh.
The testis is anchored to the region of the inguinal canal by the enlargement of the gubernacular bulb: the “swelling reaction”. This occurs between the 10th and 15th week of gestation in humans. The gubernacular bulb enlarges rapidly beyond the external inguinal ring, and anchors the intra-abdominal testis close to the inguinal region as the abdomen lengthens. The gubernacular bulb enlarges by deposition of ground substance, but also as a result of increased cell division in some species (i.e. the pig). In many mammals the diameter of the swollen gubernaculum approaches that of the testis itself. This change is due to the interaction between INSL-3 and its receptor, LGR8, which is expressed by cells within the gubernaculum. Mullerian inhibiting substance (MIS) may also be important in this process. Androgens cause regression of the cranial suspensory ligament, however, they are important later on in testicular descent.
Lag phase
In the human, there is a 10-week gap between the swelling reaction, which is completed by the 15th week of gestation, and the beginning of the inguinal–scrotal phase of testicular descent, which starts during the 7th month of gestation. So far there is no explanation as to why this pause in the descent of the testicle exists. Presumably a less obvious developmental process is occurring in the interim between the two well-described phases to allow the inguinoscrotal phase to occur. It has been proposed that the time delay allows for masculinisation of the sensory nerve roots L1 and L2 that contribute to the GFN, as this is known to be necessary for the later inguinoscrotal stage of descent in rodents. The GFN in a masculinised animal produces CGRP, and it is that which stimulates the second phase of testicular descent [21].
Inguino-scrotal phase
In the human, the testis descends through the inguinal canal at about 26 weeks of gestation, moves through the external ring at around 28 weeks, and reaches the bottom of the scrotum by 32 weeks [22]. To reach the scrotum, the gubernaculum protrudes from the body wall in a manner very reminiscent of limb bud outgrowth (Fig. 4). Both testes are usually descended at birth although in some individuals the process can be delayed for up to the 12th week of life. In rodents, the gubernaculum exhibits contractile properties during this phase. The innate contractility is similar to the cardiac myocyte. In older people, cremaster is known to be mature skeletal muscle, although not under conscious control like other skeletal muscles. However, postnatally in rats myotubes persist in the gubernaculum longer than during usual striated muscle development, which presumably confers some benefit to the descending testes.
It is probable that the proliferating cells in the tip of the gubernaculum differentiate from mesenchymal cells into myoblasts, which fuse into myotubes, moving backwards into the two layers of cremaster muscles while the tip continues proliferating. The contractions of the myotubes help move the gubernaculum through the inguinal region until the gubernacular tip cells cease proliferation at about day 10 in the rat. Outgrowth of the rat gubernaculum from the abdominal wall, as in the human, is very similar to an embryonic limb bud (Fig. 5).
Outgrowth of the rat gubernaculum during inguinoscrotal migration. The skin has been removed and pressure applied to the abdomen, which causes the free distal end of the gubernaculum to protrude in a manner reminiscent of a limb bud. CM Cremaster muscle; PV processus vaginalis; T testis; P penis; S scrotum; G gubernaculum
Gubernacular outgrowth from the abdominal wall in neonatal rats is regulated by androgens acting both directly via receptors in the bulb and indirectly via the sensory branches of the GFN. The latter release calcitonin gene-related peptide (CGRP) to stimulate growth and provide chemotactic guidance for the elongating tip [1]. Recent studies have shown that the bulb has characteristics, which are analogous to the progress zone in a limb bud, with active growth of undifferentiated mesenchyme in response to growth factors.
Various factors in limb development and their relationship to testicular descent
Hox genes
HOX genes are a subgroup of human homeobox genes that play key roles in segmental morphogenesis along the rostral–caudal body axis. There are at least 39 human HOX genes, clustered into 4 genomic groups. Gene products act as transcription factors or master regulatory switches in specifying axial identity and controlling the growth, differentiation and location of cells. Analysis of loss- and gain-of-function mutations has shown that Hox genes are important for all animals to determine cell type along the rostral–caudal axis, as well as patterning the vertebrate limb and other organ systems. Examples of the structures influenced by Hox genes include branchial arches, vertebrae, cranial nerves and ganglia, lung, gut, lower genitourinary and reproductive tracts. Many Hox gene knockout mice have been produced which has provided insight into individual Hox gene function and the functional redundancy of the Hox gene clusters. Interestingly, two individual Hox gene knockout mice have been shown to develop urogenital as well as limb malformations.
Hoxa-10
The Hoxa-10 gene is expressed in posterior domains of the developing mouse embryo. Specifically it is expressed in the posterior aspects of somite derivatives, with an expression boundary in prevertebrae 20 and 21, the neural tube and the intermediate mesoderm [23]. In the derivatives of the intermediate mesoderm, Hoxa-10 is expressed in the gut, urogenital tract, including the kidney, the mesenchyme surrounding the ureters and paramesonephric and mesonephric ducts, and the genital tubercle [24]. It is also expressed in the gubernaculum from day 15.5 of development onwards [25].
Two separate research groups have produced a Hoxa-10 gene knockout mouse with similar but not identical characteristics. A group from Boston [25] found that the male knockout mice had bilateral cryptorchidism with the location of the testes varying from perinephric to lower abdominal. A group from France [26] studied two breeds of mice and they found that in those knockout mice with a “mixed genetic background” 8/14 had unilateral cryptorchidism (in all cases this was on the left side) and 6/14 had bilateral cryptorchidism. In 5 “inbred” mice, all had bilateral cryptorchidism, and the testes were usually located near the bladder.
Both groups found that the testes in the mutant males were normal prenatally but had marked changes by adulthood, which they believed was secondary to the maldescent. The Boston group found that the gubernacular bulb was smaller in mutant males and concluded that outgrowth of the bulb and shortening of the cord had failed to occur. The French group also found the gubernacular cord in mutants to be significantly longer than normal, with a corresponding reduced distance between the kidney and the testis.
Both groups found abnormalities of the vertebrae and spinal nerves. They found an anterior homeotic transformation of L1 to T13, generating an extra T13 vertebra with an extra set of ribs. Corresponding spinal nerve changes resulted in homeotic transformation of each nerve to the closest wildtype anterior identity (the last thoracic nerve transformed to an intercostal nerve identity, the first lumbar nerve transformed to a last thoracic nerve identity, and the second to fifth nerves transformed to first to fourth lumbar nerve identity).
Although Hoxa-10 is expressed in the proximal parts of the developing forelimb and hindlimb, the alterations detected in the Hoxa-10 knockout mouse are essentially limited to the hindlimbs. These alterations are restricted to the proximal hindlimb, which encompasses the stylopod and knee region. The authors hypothesized that the cryptorchidism could be related to incorrect specification of the GFN motoneurons [26], but presumably sensory could be affected as well, as well as the possibility of an abnormal target tissue innervation.
An analysis of cryptorchid boys for anomalies within the HOXA10 gene [27] revealed several DNA polymorphisms in exon 1 in controls (n = 16) and boys with cryptorchidism (n = 45), suggesting that mutations in the HOXA10 gene might be present in some boys with cryptorchidism. However, in another study of 18 boys with cryptorchidism and 28 controls [28] no significant differences were found in polymorphisms between the control and experimental groups. The precise role of Hoxa-10 in gubernacular development remains to be determined.
Hoxa-11
The Hoxa-11 gene is also expressed widely in the developing embryo [29]. There is uniform expression in the lower arm and leg, specifically in the immature mesenchymal cells surrounding the cartilaginous precursors to developing long bones. Hoxa-11 is expressed around the Mullerian duct in males and the Wolffian duct in females, but not actually within the ducts, the distal epididymis, vas deferens and glans penis [30]. It also shows some expression in the gut, kidney and skin.
Hoxa-11 gene knockout mice have been produced and both male and females were sterile [29]. While the Hoxa-11 mutant females produced ova, they had a defective uterine environment resulting in embryo reabsorption. Hoxa-11 mutant males had bilateral cyptorchidism although the degree of maldescent was variable. The lumen of the vas deferens was small and became increasingly coiled towards the junction with the epididymis. This appears to be a partial homeotic transformation of the vas deferens into epididymis. Altered spermatogenesis was also observed but not in 100% of mutant mice. It seems likely that this is secondary to incomplete testicular descent as early orchiopexy restores fertility in mutant mice [31].
Hoxa-11 mutant mice exhibited double homeotic transformations in the spine, with the thirteenth thoracic segment posteriorized to form an additional first lumbar vertebra and with the sacral region anteriorized, forming another lumbar segment. Limb abnormalities were also observed. In mutant forelimbs, the ulna and radius were misshapen and in mutant hindlimbs the tibia and fibula were joined incorrectly and malformed at their distal ends. There were also abnormalities of the carpal and tarsal bones [32]. This alteration in vertebrae is likely to impact on spinal nerve morphology, and potentially affect the GFN.
FGF family
Fibroblast growth factors are signalling molecules that act to promote the growth and differentiation of cells. The fibroblast growth factor family contains at least 22 members and are expressed in many areas throughout the developing embryo. Fgf4, Fgf8, and Fgf10 are all expressed in the limb bud and have been extensively studied in both mouse and chick.
In the limb bud Fgf8 is expressed in the AER and its expression is required for the maintenance of limb bud outgrowth [33]. Due to its key role in gastrulation within the primitive streak the Fgf-8 knockout mouse dies during early embryogenesis [34]. Fgf8 expression has also been documented in the developing face, brain, heart, kidney and genital tubercle. The first name for Fgf8 was androgen-induced growth factor and so Fgf8 expression may be related to the presence of androgens, but it is not known whether Fgf8 plays a role in testicular descent.
Fgf10 is expressed in the mesenchyme of the limb bud and initiates and maintains expression of Fgf8 in the AER. Fgf10 is also involved in lung development and genital tubercle formation [35]. Fgf10 knockout mice die at birth due to the lack of lung development so presence of testicular descent has not been determined [9].
Fgf4 is expressed in the AER and is important in limb bud outgrowth. It is also expressed in the brain, somites, and branchial arches in chicks. As with Fgf8, Fgf4 appears to play a role in gastrulation and Fgf4 knockout mice also die during early embryogenesis [36].
Wnt genes
Wnt genes encode secreted glycosolated factors that have a range of functions in the developing embryo. There are at least 22 members of the Wnt family in vertebrates and they have many roles affecting cell proliferation, migration and polarity [37].
Wnt7a is expressed in the flanking ectoderm of the trunk prior to limb bud outgrowth and later is distributed uniformly throughout the dorsal limb ectoderm [20]. It is also expressed in the Mullerian ducts and developing brain [38]. Loss of function of Wnt7a results in ventralisation of the limbs but mutants are otherwise was fully viable. Both male and female are sterile with infertility due to failure of Mullerian duct regression and defects in uterine patterning. In mutant males the Mullerian ducts do not regress and seem to prevent the vas deferens from being complete distally, although the testis and Wolffian duct derivatives appear normal [39]. Defects in brain development have also been noted.
Wnt5a is expressed in the caudal end of the early embryo. It is not expressed in the somites but in the primitive streak and the presomitic mesoderm [40]. Later it is expressed in the tailbud, hindgut endoderm, facial primordia, developing brain and female reproductive tract [41]. In the limb and genital tubercle the expression of Wnt5a is graded with higher levels of expression occurring distally. Loss of Wnt5a function leads to perinatal lethality. Analysis of late embryonic stages showed that both forelimbs and hindlimbs were significantly shortened and lacked digits, and the genital tubercle was absent [40]. It is not yet known whether Wnt5a is involved in the process of testicular descent.
Conclusions
Both the vertebrate limb bud and the gubernaculum appear to grow from their distal tip, which contains a cluster of undifferentiated, proliferating mesenchymal cells, known in the limb bud as the progress zone. In the limb bud, as well as the branchial arches, gut, lung, and genital tubercle, the regulatory genes controlling outgrowth are highly conserved across many species. This high level of conservation during evolution suggests that the regulation of the growing “progress zone” in the distal gubernaculum is likely to be very similar to that seen in the limb bud and other outgrowths from the embryo. The abnormal gubernacular development seen with Hoxa-10 and Hoxa-11 mutations supports the concept of common regulators, but the other genes involved remain to be determined.
References
Hrabovszky Z, Pilla ND, Yap T, Farmer PJ, Hutson JM, Carlin JB (2002) Role of the gubernacular bulb in cremaster muscle development of the rat. Anat Record 267:159–165
Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA et al (2001) Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol 236(2):421–435
Mic FA, Haselbeck RJ, Cuenca AE, Duester G (2002) Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129(9):2271–2282
Gibson-Brown JJ, Agulnik SI, Silver LM, Niswander L, Papaionnou VE (1998) Involvement of T-box genes Tbx2–Tbx5 in vertebrate limb specification and development. Development 125:2499–2509
Saito D, Yonei-Tamura S, Kano K, Ide H, Tamura K (2002) Specification and determination of limb deformity: evidence for inhibitory regulation of Tbx gene expression. Development 129:211–220
Mariani FV, Martin GR (2003) Deciphering skeletal patterning: clues from the limb. Nature 423(6937):319–325
Dudley AT, Ros MA, Tabin CJ (2002) A re-examination of proximodistal patterning during vertebrate limb development. Nature 418:539–544
Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, Murtaugh LC et al (1996) Analysis of Hox gene expression in the chick limb bud. Development 122:1449–1466
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T et al (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21(1):138–141
Ohuchi H, Nakagawa T, Itoh N, Noji S (1999) FGF10 can induce Fgf8 expression concomitantly with En1 and R-fng expression in chick limb ectoderm, independent of its dorsoventral specification. Dev Growth Differ 41(6):665–673
Sun X, Mariani FV, Martin GR (2002) Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418(6897):501–508
Boulet AM, Moon AM, Arenkiel BR, Capecchi MR (2004) The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol 273(2):361–372
Tickle C (2002) Molecular basis of vertebrate limb patterning. Am J Med Genet 112(3):250–255
Stratford T, Horton C, Maden M (1996) Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr Biol 6(9):1124–1133
Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C et al (2000) A model for anteroposterior patterning of the vertebrate limb based on sequential long-and short-range Shh signalling and Bmp signalling. Development 127:1337–1348
Zakany J, Kmita M, Duboule D (2004) A dual role for Hox genes in limb anterior–posterior asymmetry. Science 304(5677):1669–72
Deschamps J (2004) Developmental biology. Hox genes in the limb: a play in two acts. Science 304(5677):1610–1611
Chen H, Johnson RL (2002) Interactions between dorsal–ventral patterning genes lmx1b, engrailed-1 and wnt-7a in the vertebrate limb. Int J Dev Biol 46(7):937–941
Pizette S, Abate-Shen C, Niswander L (2001) BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development 128(22):4463–4474
Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D–V and A–P axes of mouse limb. Nature 374(6520):350–353
Hutson JM, Hasthorpe S (2005) Testicular descent and cryptorchidism: the state of the art in 2004. J Pediatr Surg 40(2):297–302
Heyns CF, Hutson JM (1995) Pediatric urology: review article: historical review of theories on testicular descent. J Urol 153(3):754–767
Haack H, Gruss P (1993) The establishment of murine Hox-1 expression domains during patterning of the limb. Dev Biol 157(2):410–422
Benson GV, Nguyen TH, Maas RL (1995) The expression pattern of the murine Hoxa-10 gene and the sequence recognition of its homeodomain reveal specific properties of Abdominal B-like genes. Mol Cell Biol 15(3):1591–1601
Satokata I, Benson G, Maas R (1995) Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374(6521):460–463
Rijli FM, Matyas R, Pellegrini M, Dierich A, Gruss P, Dolle P et al (1995) Cryptorchidism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc Natl Acad Sci 92:8185–8189
Kolon TF, Wiener JS, Lewitton M, Roth DR, Edmond T. Gonzales J, Lamb DJ (1999) Analysis of homeobox gene Hoxa10 Mutations in cryptorchidism. J Urol 161:275–280
Bertini V, Bertelloni S, Valetto A, Lala R, Foresta C, Simi P (2004) Homeobox HOXA10 gene analysis in cryptorchidism. J Pediatr Endocrinol Metab 17(1):41–45
Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K et al (1995) Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 121:1373–1385
Bomgardner D, Hinton BT, Turner TT (2003) 5′ Hox Genes and Meis 1, a Hox-DNA binding cofactor, are expressed in the adult mouse epididymis. Biol Reprod 68:644–650
Lewis AG, Pecha BR, Smith EP, Gardner BJ, Hsieh-Li HM, Potter SS et al (2003) Early orchiopexy restores fertility in the Hoxa 11 gene knockout mouse. J Urol 170(1):302–305
Small KM, Potter SS (1993) Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev 7(12A):2318–2328
Moon AM, Capecchi MR (2000) Fgf8 is required for outgrowth and patterning of the limbs. Nat Genetics 26:455–459
Crossley PH, Martin GR (1995) The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121:439–451
Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M et al (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127(11):2471–2479
Shamim H, Mason I (1999) Expression of Fgf4 during early development of the chick embryo. Mech Dev 85(1–2):189–192
Miller JR (2002) The Wnts. Genome Biol 3(1):REVIEWS3001
Parr BA, Shea MJ, Vassileva G, McMahon AP (1993) Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119:247–261
Parr BA, McMahon AP (1998) Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395(6703):707–710
Yamaguchi TP, Bradley A, McMahon AP, Jones S (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126(6):1211–1223
Miller C, Pavlova A, Sassoon DA (1998) Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 76(1–2):91–99
Author information
Authors and Affiliations
Corresponding author
Additional information
Jenny Huynh, Natalie S. Shenker and Sophie Nightingale made equal contributions to the paper.
Rights and permissions
About this article
Cite this article
Huynh, J., Shenker, N.S., Nightingale, S. et al. Signalling molecules: clues from development of the limb bud for cryptorchidism?. Pediatr Surg Int 23, 617–624 (2007). https://doi.org/10.1007/s00383-007-1907-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-007-1907-9