Abstract
Significant side effects are correlated with bladder augmentation. Recently, small intestinal submucosa (SIS) has been proposed for clinical use. The efficacy of SIS bladder regeneration was studied in a porcine experimental model. Partial cystectomy (40–60% of bladder wall) was performed and replaced by SIS graft. Animals were planned to be killed at 2 weeks, 5 weeks and 3 months. Bladder capacity at 40 cmH2O pressure and macroscopic graft morphology were assessed before and after SIS implant. Histological examination was carried out with computer assisted morphometric analysis for collagen/smooth muscle ratio. Student’s t test was adopted for statistical analysis. Two piglets died on the 9th and 10th post-operative day due to urinary peritonitis. The remaining piglets were killed after uneventful post-operative period at 5 weeks (two animals) and 3 months (two animals). The bladder capacity was reduced (−18%) at the 5 week follow-up and quite similar to the pre-operative volume (+2.5%) at the 3 months control. No diverticular formation, bladder calculi, mucus and urinary infection were found. The SIS graft resulted not significantly contracted. Histology at 10 days showed SIS membrane lined by transitional epithelium islands with some capillaries. At 5 weeks, transitional epithelium was fully covering the graft; new blood vessels and fibroblasts with smooth muscle cells were observed. At 3 months, the SIS was not evident. Two layers were defined: inner transitional epithelium, outer collagen with fibroblasts and muscular bundles. Computer assisted morphometric analysis showed collagen/muscle ratio 70/30% (normal bladder=56/44%, P<0.05). The SIS was effective as a scaffold for bladder wall regeneration in four out of six animals. Long-term studies are required to confirm the efficacy of the newly developed wall and for eventual clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reconstruction of the human bladder is still one of the great surgical challenges in adult and pediatric urology. Different materials and techniques have been experimentally and clinically described, with most of these using segments of gastro-intestinal tract. However, the use of bowel is associated with significant morbidity and functional alteration [1]. Alloplastic biomaterials [2] and different operative procedures, such as autoaugmentation [3], have been proposed to preserve the transitional urothelium, with discouraging or not-satisfying results.
The ideal procedure for bladder augmentation should be easy to perform without bowel and peritoneal cavity violation and should guarantee good functional results at long-term follow-up [2, 4]. In the last decade, Kropp and his research group from Indianapolis evaluated the use of small intestinal submucosa (SIS), harvested from the submucosal layer of porcine intestine, as a possible bladder augmentation material, acting as a bioscaffold for tissue regeneration in vivo in a series of experimental models in rats and dogs [4–6]. Their SIS-regenerated bladder demonstrated good compliance and capacity achievement, with contractile activity and radiological and histological results similar to native bladder [7].
The SIS is a xenogenic membrane, harvested from the pig’s small intestine, obtained when the tunica mucosa is removed from the inner surface, and the serosa and tunica muscularis are removed from the outer surface. The acellular, collagen-rich membrane has been demonstrated to be non-immunogenic and to stimulate rapid replacement by native tissues, as arterial and venous graft [8, 9]. More recently, the SIS has been proposed for clinical use and manufactured by Cook Urological Co® (Spencer, IN, USA), obtaining FDA and CE approval (Surgisis® ES). The aim of the study was to prove the biological and histopathological characteristics of the commercially available SIS as a template biomaterial for bladder regeneration, stimulating the urothelium, new vascularization, smooth muscle and peripheral nerve development in a porcine model of bladder augmentation.
Materials and methods
Surgical procedure for SIS augmentation
Six female Landrace piglets, aged 2 months, body weight 15 to 18 kg, received general anesthesia with 4% isoflurane per endotracheal tube, with spontaneous ventilation. The abdomen and genitalia were prepared with povidone-iodine scrub and draped in a sterile fashion. The animal experiments were performed considering the “Principles of laboratory animal care”.
The bladder capacity was assessed filling the bladder with warm saline through a suprapubic cystostomic cannula at the pressure of 40 cm H2O (Table 1). The abdomen was opened through an infraumbilical midline incision and the bladder exposed. Partial cystectomy was performed, resecting the bladder dome with the anterior wall and saving the posterior wall, trigone and bladder neck: the resected bladder wall was measured, corresponding to 45 to 60% of the detrusor surface (Table 1). The bladder was replaced by a multi-layered SIS graft (Surgisis® ES, Cook® Ireland Ltd, Limerick, Ireland), prepared in a rhomboid fashion with dimension similar to the resected bladder wall portion and oriented with the stratum compactum surface, corresponding to the original mucosal surface, facing the bladder lumen. The graft was sewn using polyglactin 5/0 watertight running suture and divided into four segments. Permanent marking sutures were placed at the four corners around the sutured SIS augmentation graft at the 3, 6, 9 and 12 o’clock positions. The SIS graft was covered with a strip of omentum or perivesical fat, before the abdominal wall closure. The augmented bladder was filled with saline through a cannula at 40 cm H2O pressure and the new capacity was assessed at time 0 (T0, Table 1). The pigs received antibiotics for 4 days and were allowed to void spontaneously. Animals were planned to be randomly killed after 2 weeks (T1), 5 weeks (T2) and 3 months (T3). Before the killing, the bladder capacity at 40 cm H2O and bladder wall compliance with detrusor activity were assessed under general anesthesia, through an 18 gauge suprapubic catheter. The bladder was harvested for macroscopical and histological examination: routine sections were stained with hematoxylin-eosine, and Masson trichrome was used for assessment of connective tissue; immunohistochemical study was stained with smooth muscle actin for smooth muscular fibers, S100 protein for nerve trunks, CD34 for endothelial cell of vessels were performed with PAP technique. A computer assisted morphometric analysis was also performed, for collagen/smooth muscle ratio. A total number of 12 to 16 images was analyzed for each specimen at ×50 magnification and computerized for morphometry using image software. The areas occupied by smooth muscle and collagen were circumscribed, and the surfaces enclosed in the computer assisted line drawing were calculated in percents by the software.
Statistical analysis of two independent variables was performed by the Fisher 2-sided exact test, considering P<0.05 as statistically significant.
Results
Two piglets died on the post-operative 9th and 10th day (T1): at autopsy urinary peritonitis was found, due to dehiscence at the suture line between the SIS implant and the native bladder wall. The remaining four piglets (66.6%) had uneventful post-operative period and were randomly killed at 5 weeks (T2 = two animals) and 3 months (T3 = two animals) after from the initial bladder surgery. Bladder capacity at 40 cm H2O filling pressure was reduced (−18%) at 5 weeks follow-up and not significantly modified (+2.5%) at time 3 control, compared with the time 0 situation (Table 2). The bladder compliance was found reduced at time 2 cystometry, but became normal at 3 months interval (Table 2).
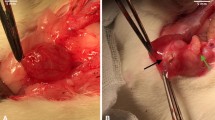
At macroscopical examination (T2 and T3) the “new” bladder wall was uniformly filled without evidence of diverticular development at the graft size. It was covered by a soft, well vascularized, connective tissue. At cystectomy, no bladder calculi, mucus and urinary infection were found at 1 and 3 months interval from SIS augmentation. The four permanent marking sutures left at the time of SIS application were easily identified at the implant corners (Fig. 1). The SIS augmentation graft resulted not significantly contracted from initial measures (Table 2).
Histology showed different pictures, considering the interval time from initial surgery. At time 1, the SIS membrane was well recognized and lined by islands of thin transitional epithelium. New capillaries were not yet observed from the external aspect of the implant. At 5 weeks interval (T2), the inner transitional epithelium was fully covering the membrane that was poorly recognized at the central portion of the implant. Newly formed blood vessels and small capillaries were developed from the native bladder wall towards the central portion of the previously implanted SIS. A significant centripetal ingrowth of fibroblasts was observed, with minor presence of newly formed smooth muscle cells (Fig. 2a). At 3 months (T3), no more SIS membrane was evident also in the central portion of the implant. The new vascularization was more evident together with an almost thick and well organized newly developed bladder wall. Three layers were grossly recognized. The transitional epithelium was uniformly distributed on the full inner graft surface (Fig. 2b). The central layer presented well-developed small blood vessels, collagen fibers, linearly organized with several interspersed fibroblasts (Fig. 2c). Thin smooth muscle fibers with focal aggregation in small muscular bundles were properly stained and recognized with smooth muscle actin (Fig. 2d). Small nervous fibers between the muscle fibers were evident, using with S100 protein immunohistochemical stain (Fig. 2e). Endothelial lining of vessels were marked with CD34. The other part of bladder wall was lined by a young, well-vascularized connective tissue, as maturative evolution of the omental flap outside the SIS membrane.
a (time 3) The transitional epithelium is fully covering the inner surface, with the SIS membrane poorly recognizable (collagen tissue) with regular migration of smooth muscle fibers (hematoxylin-eosin staining: 5×). b (time 3) Smooth muscle fibers proliferation in parallel sheets into the loose vascularized connective tissue (hematoxylin-eosin staining: 20×). c (time 3) Masson trichrome staining: differently stains smooth muscle fibers into the loose connective tissue (MT: 20×). d (time 3) Newly formed smooth muscle fibers marked by immunohistochemical technique with smooth muscle actin (SMA: 20×) e (time 3) Small nerve trunks between smooth muscle fibers (S100 protein: 60×)
The computer assisted morphometric analysis showed the collagen/muscle tissue ratio presenting wide presence of collagen in the newly developed bladder wall (72% collagen vs. 28%) muscular tissue. The collagen/muscular distribution in the native bladder wall of the same animals was 56% collagen versus 44% muscular fibers (Fig. 3). The difference of musculature distribution in the newly developed bladder wall versus native bladder wall was statistically significant (P<0.05).
Discussion
Bladder augmentation or partial substitution is often required for different clinical situations both in adult and pediatric urology, as bladder exstrophy, posterior urethral valves, neurogenic bladder, bladder fibrosis and oncology. The side effects and the complications related to the use of gastro-intestinal tract (stomach, ileum, cecum, sigmoid) are well known [1, 10]. On the other hand, autoaugmentation techniques and the use of alloplastic materials to increase bladder capacity and compliance showed frequently discouraging and not satisfying results [2, 11].
Ideally, any surgeon would like to adopt a reconstructive procedure for bladder augmentation and partial substitution which should be easy to perform, without violation of the peritoneal cavity and without use of any gastro-intestinal tract. The use of bowel exposes to significant side effects, as the metabolic consequences, the mucus and stones formation, the risk of frequent urinary tract infections and of perforation of the augmented bladder. The carcinogenetic risk should be considered, as well, at long-term follow-up. The ideal surgical procedure should avoid those consequences and present low complication rate and ensure good functional results in terms of increase of bladder capacity and compliance as well as outlet efficacy to the patients. These urodynamic results should remain stabile at long-term follow-up.
The principle of bladder regeneration is not a new concept, as two Italian researchers published it in the late nineteenth century [12]. The possibility of bladder tissues regeneration seems very attractive to the urologist who is faced with the need of increasing the bladder storage activity. Two different strategies have been proposed in the last few years to achieve the goal: in vitro, adopting the tissue engineering techniques stimulating the cellular growth on biodegradable materials as scaffolds, with the use of specific grow-factors to stimulate cultured urothelial and smooth muscle cells before transplanting the engineered tissues on the host [13, 14].
The second strategy to stimulate bladder tissues regeneration is in vivo, promoting the cellular ingrowth directly from the native bladder wall. After a preliminary report [15], the group of researchers from Indiana University [5, 6] proposed the use of a membrane of SIS, harvested from the pig bowel, that was acting as a bioscaffold for stimulating the tissue regeneration. The authors demonstrated the possibility to achieve regeneration of the main components of the bladder wall (the transitional epithelium and the smooth muscular layer) in a series of experimental models carried on rats and dogs, utilizing a membrane of acellular submucosa harvested experimentally from the porcine small bowel [4, 6, 16]. The initial experimental results on animal models were presented almost successful; the submucosa was found to act as a bioscaffold in vivo, stimulating the tissue regeneration on the host animals. The authors could achieve bladder wall regeneration after partial cystectomy in their experimental animal models: the “SIS-regenerated” bladder was demonstrated to have good anatomical and functional properties, achieving almost normal bladder volume after partial cystectomy with good compliance and viscero-elastic property [17]. The new bladder wall showed significant newly developed vascularization, with some ingrowth of thin nerves within a well organized fibro-muscular layer [18].
Recently, the submucosa harvested from the piglet bowel has been proposed for several clinical uses, manufactured by Cook Urological Co (Spencer) as Surgisis®. It is a xenogenic, collagen-rich and acellular membrane, harvested from the pig small intestine. The material has been demonstrated to be not immunogenic and able to act as a scaffold to stimulate cellular regeneration [4, 15]. The aim of our experimental model was to verify the results of in vivo cellular and bladder tissues regeneration, using the Surgisis® membrane as template to stimulate rapid ingrowth of the native bladder wall. In the experimental study, six piglets were used, as the non-immunogenicity of the SIS was previously demonstrated [6, 15]. The experimental work-up was planned performing 40 to 60% partial native cystectomy (Table 1). The outcome was randomized at three different intervals from the initial surgery. The bladder capacity at 40 cm H2O filling pressure became not significantly different from the pre-operative status at T3 (+2.5%, P = NS). The macroscopical examination at T2 and T3 showed absence of diverticular development on the SIS-augmented portion of the bladder, that was covered outside by soft, well vascularized connective tissue. The previously implanted SIS graft, which was demonstrated to be not significantly shrunk from the initial diameters (−10%; Fig. 1).
The histological findings showed progressive regeneration of the bladder wall along the time. At T1, the SIS membrane was well recognized and islands of transitional epithelium were observed lining the inner surface. At T2, the transitional epithelium was observed to fully cover the inner face of the SIS membrane that was poorly recognized only at the central implant portion. New blood capillaries were running from the external aspect of the implant towards the central portion. A significant ingrowth of fibroblasts was observed centripetally, with few smooth muscular cells. At T3 follow-up, no more SIS membrane was evident and the new vascularization was well developed inside the newly formed bladder wall. It was observed as almost thick and organized in three different layers: The inner surface, consisting in thin transitional epithelium. The central layer, represented by loosely textured connective tissue containing fibroblasty and rich in smooth muscular actin positive muscular fibers arranged in multi-layered fashion parallel to the mucosal surface. Small nerve trunks are present between muscular fibers. A well vascularized connective tissue represented the other covering layer.
The computer assisted morphometric analysis showed a significantly reduced smooth muscle/collagen ratio on the regenerated bladder wall (Fig. 3). It could be supposed that a 3 month interval time is too short to permit the complete regeneration of the elastic and muscular component of the bladder wall, with adequate neurofibrillar supply. Some experimental experiences seem to confirm the progressive ingrowth of fibromuscular cells with inflammatory reaction and some calcifications or osseous metaplasia [7, 19, 20]. The Surgisis® graft seemed to shrink moderately (−10%) in the first period, probably as consequence of the lack of enough elastic tissue. A concern to the clinical use of the Surgisis® membrane to bladder augmentation arises from the risk that the regenerated bladder wall could repeat the histopathological abnormality of the native bladder: for example the neurogenic bladder or the poor-compliant fibrotic wall, as we observe in the extrophic or post-urethral valves bladders.
In conclusion, the new commercially available SIS membrane resulted as effective to act as scaffold for “imperfect” bladder wall regeneration in four out of six animals of the present experimental study. The urinary peritonitis in the two cases could be attributed to the lack of bladder drainage in the pig experimental model. The bladder wall regeneration starts from the inner transitional epithelium and continues through smooth muscle bundles, in a centripetal fashion. Newly developed vascularization and peripheral nerves ingrowth are present with an organized fibro-muscular layer, but muscular/connective ratio was found lower than normal in some fibrotic tissue developed on the graft. Our results could be considered as encouraging, and similar to those obtained using fresh harvested porcine intestinal submucosa membrane in other similar experimental studies [5, 7, 16].
Recently, Chung et al investigated the “in vivo” benefits of seeding stem cells into SIS for bladder augmentations and demonstrated increased collagen formation at 3 months. However, the experimental study was not a long-term follow-up to evaluate the histological and functional properties, which are characteristic of bladders using unseeded SIS [21].
Urologists would like to have an alternative to the use of bowel, but as yet the manufactured SIS membrane does not appear to guarantee completely good results [20, 22]. Long-term studies are required to confirm the clinical use of SIS membrane as a scaffold for bladder wall regeneration in humans [23]. The risk of carcinogenicity must be rolled out and the long-term stabilization of the viscero-elastic properties of the newly developed wall has to be confirmed. Research perspectives on this field could be promising, with the use of growth factors (TGF-β), stem cells and myoblasts within the SIS graft in vivo applied, to enhance and modulate the autologous regeneration process of the bladder wall [14].
References
Mitchell ME, Gonzales R, Cabral BH, Bauer SB, Gearhart JP, Filmer RB (1987) Bladder augmentation problems in neurovesical dysfunction. Dial Pediatr Urol 10:1
Gleeson MJ, Griffith DP (1992) The use of alloplastic biomaterials in bladder substitution. J Urol 148:1377
Cartwright PC, Snow BW (1989) Bladder autoaugmentation: partial detrusor excision to augment the bladder without the use of bowel. J Urol 142:1050
Kropp BP, Pope JC IV (1997) Small intestinal submucosa: a novel substance for the study of cellular interaction and regeneration in the bladder. Dial Pediatr Urol 20 10:2
Kropp BP, Eppley BL, Prevel CD, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA (1994) Experimental assessment of Small Intestine Submucosa as a bladder wall substitute. J Urol 151:501
Kropp BP, Eppley BL, Prevel CD, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA (1995) Experimental assessment of Small Intestine Submucosa as a bladder wall substitute. Urology 46(3):396
Pope JC IV, Davis MM, Smith ER Jr, Walsh MJ, Ellison PK, Rink PC, Kropp BP (1997) The ontogeny of canine Small Intestinal Submucosa Regenerated Bladder. J Urol 158(3):1105–1110
Lantz GC, Badylak SF, Coffey AC, Gaddes LA, Sandusky GE (1990) Small Intestinal Submucosa as a small-diameter arterial graft in the dog. J Invest Surg 3:217
Lantz GC, Badylak SF, Coffey AC, Gaddes LA, Sandusky GE (1992) Small Intestinal Submucosa as a superior vena cava graft in the dog. J Surg Res 53:175
Gonzales R, Buson H, Ried C, Reinberg Y (1994) Seromuscolar colocystoplasty lined with urothelium: experimental study. Urology 44:743
Marte A, Di Meglio D, Cotrufo AM, Di Iorio G, De Pasquale M, Vessella A (2002) A long-term follow-up of autoaugmentation in myelodysplastic children. BJU Int 89(9):928
Tizzoni G, Poggi A (1898) Die Wiederherstellung der Harnblase: experimentelle Untersuchungen. Zcentrabl Chir 15:921
Atala A (2004) Tissue engineering for replacement of organ function in the genito-urinary system. Am J Transplant 6(Suppl 4):58–73
Zhang Y, Kropp BP, Lin HK, Cowan R, Cheng EY (2004) Bladder regeneration with cell-seeded small intestinal submucosa. Tissue Eng 10(1–2):181–187
Kropp PM, Lingeman JE, Siegel YL, Badylak SF, Demeter RJ (1994) Biocompatibility fo Small Intestinal Submucosa in urinary tract as augmentation cystoplasty graft and injectable suspension. J Endourol 8:125
Kropp BP, Rippy MK, Balylak SF, Adams MC, Keating MA, Rink RC, Thor KB (1996) Regenerative urinary bladder augmentation using Small Intestinal Submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentation. J Urol 155(6):2098
Kropp BP, Sawyer BD, Shannon HE, Rippy MK, Balylak SF, Adams MC, Keating MA, Rink RC, Thor KB (1996) Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation in vitro compliance and contractility. J Urol 156(25):599–607
Vaught JD, Kropp BP, Sawyer BD, Rippy MK, Badylak SF, Shannon HE, Thor KB (1996) Detrusor regeneration in the rat using porcine Small Intestinal Submucosal graft: functional innervation and receptor expression. J Urol 155(1):374–378
O’Conner RC, Patel RV, Steinberg GD (2001) Successful repair of a uretero-neobladder stricture using porcine small intestine submucosa. J Urol 165(6):1995
Paterson RF, Lifshitz DA, Beck SD, Siqueira TM Jr, Cheng L, Lingeman JE, Shalhav AL (2002) Multilayered small intestinal submucosa is inferior to autologous bowel for laparoscopic bladder augmentation. J Urol 168(5):2253–2257
Chung SY, Krivorov NP, Rausei V, Thomas L, Frantzen M, Landsittel D, Kang YM, Chon CH, Christopher S, Fuchs G (2005) Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol 174(1):353–359
Rink RC, Kropp BP (2003) Personal communication
Metwalli AR, Colvert Jr III, Kropp BP (2003) Tissue engineering in urology: where are we going? Curr Urol Rep 4(2):156–163
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caione, P., Capozza, N., Zavaglia, D. et al. In vivo bladder regeneration using small intestinal submucosa: experimental study. Ped Surgery Int 22, 593–599 (2006). https://doi.org/10.1007/s00383-006-1705-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-006-1705-9