Abstract
Despite advancements in catheter design and dialysis technique, catheter related complications still remain a common clinical problem in paediatric patients on chronic peritoneal dialysis (PD); in particular, infections are a common cause of patient’s morbidity and technique failure. In the present paper, data on 89 catheters implanted between January 1986 and December 2002 are reviewed to analyse the major causes of complications and/or PD failure and to ascertain their optimal management. A total of 89 catheters were implanted in 78 patients at the start of chronic PD: 26 in children under 2 years of age, 14 in children aged 2–5 years and 49 in patients over 5 years. Mean age of patients was 76.1 ± 73.0 months and median treatment time 14.5 ± 13.1 months. All catheters were surgically implanted and partial omentectomy was performed in 70% of cases. Straight Tenckhoff catheters were used in 70 cases (78%), curled ones in 19 (22%). Sixty-three catheters (71%) had two cuffs, 26 (29%) a single cuff. The entry-site was the midline in 34 patients (38%) and the paramedian line in 55 patients (62%). Catheter survival rate was 80% at 12 months, 62% at 24 months and 58% at 36 and 48 months, respectively. The incidence of catheter-related complications was one episode every 6.4 PD-months, and they were mainly represented by peritonitis (61%), exit-site infections and tunnel infection (ESI + TI: 23%), catheter obstruction (5%), dislocation (3.5%), leakage (2.5%). After the introduction of curled single-cuff catheters, a considerable reduction in the peritonitis incidence was observed during the last 7 years. A more prolonged catheter survival was observed in older children (>5 vs. <2 years: P < 0.05). Leakage was less common in catheters with paramedian entry-site compared with catheters implanted on the midline. In 7 out of 11 (64%) patients with catheter obstruction, omentectomy had not been performed. Single-cuff catheters had a lower infection-rate than double-cuff catheter (P < 0.01). Single cuff-curled Tenckhoff catheter can be considered the first choice catheter. Single cuff-catheters are not associated with an increase of infections. The surgical technique requires a strict adherence to a standardized procedure and a dedicated team, in order to obtain a reduction of the complications, a prolonged catheter duration and a better quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 2 decades chronic peritoneal dialysis (PD) has emerged as the first choice pediatric dialysis modality. Despite the improvements in catheter survival, tunnel infection (TI), exit-site infection (ESI), and peritonitis remain the most common causes of morbidity, sometimes leading to treatment failure [1–5]. Over 2.5% of children on PD are reported to have had TI or ESI within the first 6 months of PD [6] with a consequent doubling of the risk of peritonitis [3]. A detailed knowledge of the complications is of paramount importance to guide our efforts towards improving patient’s care and quality of life. Aim of the present study is to review the experience of 17 years (1986–2002) in PD, mainly from a surgical perspective, and to analyse the major causes responsible for catheter-related morbidity.
Patients and methods
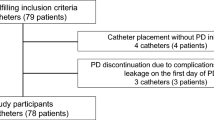
From January 1986 to December 2002, 89 catheters were implanted in 78 patients (48 males) with end-stage renal failure due to the following primary nephropathies: glomerulopathies 30 (39%), renal hypodysplasia with or without urologic abnormalities 23 (28%), hereditary nephropathies 17 (22%), renal tumours 4 (5%) and unknown 4 (6%). Their mean age was 76.1 ± 73.0 months and the mean treatment duration was 14.5 ± 13.1 months, with a total of 1,291 patient–months analysed.
Table 1 shows the type of catheter used, the number of cuffs, the site of insertion, the exit-site location and the course of the tunnel. All catheters, for home PD, were surgically implanted under general anaesthesia, in open technique, by the same team of surgeons. Partial omentectomy was performed in 70% of cases. In particular, while in the first period this procedure was not systematically obtained, in the last years all patients underwent omentectomy, except selected cases, such as children with scarce omental tissue. Starting from 2000, straight catheters were substituted by curled catheters. After catheter implantation, the patients were regularly followed by the pediatric nephrology unit and addressed to Automated Peritoneal Dialysis, with different treatment schedules according to individual needs. In the present paper, ESI, TI and peritonitis were defined according to the International Society for Peritoneal Dialysis guidelines/recommendations [7]. ESI is diagnosed in the presence of purulent discharge from the sinus tract, or marked pericatheter swelling, redness, and/or tenderness with or without a pathogenic organism cultured from the exit site; TI is defined as presence of pain and signs of inflammation along the subcutaneous tunnel, not necessarily associated with ESI; empiric diagnosis of peritonitis is made if two of the following criteria are present: (1) the peritoneal effluent is cloudy, the effluent white blood cell (WBC) count is greater than 100 mm−3 and at least 50% of the WBCs are polymorphonuclear leukocytes; (2) symptoms of peritonitis, like abdominal pain and fever, are detectable; (3) culture of peritoneal fluid is positive for micro-organisms [8].
Data are shown as mean ± standard deviation or median and range, depending on whether normally distributed or not. Statistical analysis was carried out by means of the ANOVA or the Mann–Whitney test for unpaired data, as applicable. Contingency tables were analysed by means of the chi-square. A probability lower than 0.05 was considered significant.
The cumulative probability of catheter survival was estimated by the Kaplan–Meier life-table analysis, with catheter removal for infectious or mechanical complications as the end-point. Catheter removal for reasons unrelated to the peritoneal access (transplantation, switch to hemodialysis for patient’s choice, death for causes other then peritonitis) was considered censored data.
Results
During the observation period, PD-related surgical complications had an overall frequency of 1 event every 6.4 PD-months. The type and relative distribution of the complications are shown in Table 2; each catheter had a mean of 1.9 ± 2.2 events.
Peritonitis, ESI and TI, grouped together as infectious complications (IC), were the most common events, with a frequency of 1:7.6 months of PD; the peritonitis rate was 1:13.2 months of PD. Staphylococcus epidermidis (n = 26) and Staphylococcus aureus (n = 8) were the agents most commonly involved (Table 3).
No age-related differences, in terms of both type and incidence of IC, were found in our series; in particular, the peritonitis rate (expressed as events per patient) was as follows: children <2 years: 0.7 ± 1.0, 2–5 years: 1.4 ± 1.9 and >5 years: 1.8 ± 2.4 (P = n.s.).
Tunnel infection and ESI were more frequent (1:8.5 PD-months) than peritonitis and represented a very important source of morbidity, with a rate of 1 episode every 15.1 patient-months and 1:6.2, in single-cuff and double-cuff, respectively (P < 0.01).
As far as the tunnel course is concerned, the swan-neck one (mainly associated with downward orientation) had a rate of TI and ESI of 1:16.3 months of PD, while the straight tunnel course (mainly associated with lateral orientation) exhibited a TI and ESI of 1:6.2 (P = n.s.).
A considerable reduction in the peritonitis incidence, although not statistically significant, was observed during the last 7 years compared to earlier time. In details, during the 1986–1995 period (mean catheter life 14 months), 1.5 ± 2.3 episodes/catheter were recorded, while during the 1996–2002 period (mean catheter life 15 months) the corresponding figure was 0.9 ± 1.2 (P = n.s.).
The entry-site of the catheter (midline vs. paramedian) does not seem to have influenced the occurrence of both IC and catheter survival (Table 4). On the other hand, it was associated with an increased occurrence of early peritoneal leakage: the only five cases were all observed in catheters with a median entry-site.
Catheter obstruction occurred in 11/89 catheters; 7/11 were implanted in patients who had not undergone omentectomy (P < 0.02).
Catheter survival was 80% at 12 months, decreased to 62% at 24 months and remained stable thereafter: 58% both at 36 and 48 months (Fig. 1). The causes of catheter removal are reported in Table 5.
A more prolonged catheter survival was observed in older children, with a median (range) of 6.5 (0.2–110), 16 (5–68) and 19.5 (1–133) months, in children < 2 years, 2–5 years and > 5 years (> 5 years vs. < 2 years: p < 0.05), respectively.
Discussion
This is a retrospective study on patients who had a peritoneal catheter inserted in the Department of Pediatric Surgery and were treated with PD in the Unit of Pediatric Nephrology, Dialysis and Transplant in Milan from 1986 to 2002.
In the first 24 months catheter survival is similar to that of other pediatric studies [9–17], showing a rapid decline of the curve (62%). The first 2 years seem to represent the critical period in the life of the catheters. A possible interpretation for the non-linear pattern of the survival curve is that during the first period of PD, a selection of patients based on their adherence to procedures and prescriptions takes place and only those with higher compliance and consequent lower complication rate, remain on treatment. This observation emphasizes the importance of careful training and continuing education of patients for a successful PD program. Nevertheless individual factors (i.e. nutritional status, metabolic balance, local factors) may certainly be involved in predisposing to catheter complications, therefore affecting catheter life span.
On the contrary, in the present study a significant difference in catheter survival is observed at 3 and 4 years, as compared to other studies: 58% versus 43.8 and 34.6, respectively [17]. This may reflect the improved experience and technical skill reached by a dedicated team of a single centre.
As shown in previous reports [5], the mean catheter survival seems to be age-related, and children older than 5 years are expected to have a more prolonged catheter life. It can be speculated that either older children have a lower susceptibility to infections or the management of the catheter is simpler due to an improved patient’s cooperation.
Infectious complications are confirmed as the most common causes of catheter loss, as reported by other dialysis centres [10–11]. Peritonitis episodes accounted for 61% of all the PD complications and they were mainly sustained by Staphylococcus. This finding is not surprising, because the majority of papers addressing the issue of infections complicating PD have reported S. aureus as the leading involved microrganism [3, 12].
The significant decrease in ICs observed during the last few years (1996–2002), in comparison to the previous period (1986–1995), deserves a comment. Among the possible explanations, we identify two areas. On one hand, there is the improved skills reached by the dedicated team (pediatric nephrologists, surgeons, nurses and psychosocial workers) in catheter implantation and manipulation techniques, together with the improved training of patients and/or parents. Furthermore, in recent years a systematic change in the implantation technique took place in our Pediatric Surgery Unit, based on indications rising from the relevant literature (mainly studies of the MEPPS and of the NAPRTCS) [2–3] with paramedian insertion and downward orientation as standard procedure. During the same recent years, single-cuff curled Tenckhoff catheter became the first choice catheter in our Unit and, in contrast with the experience reported in both adult [10] and pediatric [2] studies, the use of single-cuff catheters was not associated to an increase in TI and ESI. It may be worth mentioning that, in our experience, the presence of second, distal cuff, which is, by definition near to the exit site, sometimes represented a factor responsible for persistent TI for the contamination of the cuff itself. In such cases the subcutaneous cuff had to be removed (cuff shaving).
As to the management of ESI, the standard protocol in our Unit includes the following three steps: (1) culture of ES secretion and daily disinfection with sodium hypochloride (0.05–0.15%); (2) if no improvement or in case of coexisting TI, intraperitoneal antibiotic therapy (either empirical or based on colture) is administered for at least 15 days; (3) recurrent and non responsive infections are treated with a conservative surgical management, which includes the repositioning of the subcutaneous portion into a new tunnel with a controlateral course. If a distal cuff is present this is shaved according to previously described techniques [13–15].
In our experience, non-IC (leakage, obstruction, dislocation, intestinal obstruction, inguinal hernia, hydrocele and hydrothorax) had a lower frequency as compared to other studies (16 vs. 27%) [17]. Most of these complications are related to the surgical technique. In particular omentectomy seems to prevent from catheter obstruction, expecially in younger children [16–18], while the paramedian insertion shows a lower incidence of leakage [19–21].
In conclusion, the surgical technique in the catheter implantation requires a strict adherence to a standardized procedure and a dedicated team. By this way it is possible to obtain a significant reduction in catheter complications, with prolonged catheter duration and a better quality of life for both children and parents.
Abbreviations
- PD:
-
Peritoneal dialysis
- ESI:
-
Exit-site infections
- TI:
-
Tunnel infection
- IC:
-
Infectious complications
References
Kuizon B, Melocoton TL, Holloway M, Ingles S, He-Jing, Fonkalsrud EW, Salusky IB (1995) Infectious and catheter-related complications in pediatric patients treated with peritoneal dualysis institution. Pediatr Nephrol 9:12–17
Schaefer F, Klaus G, Muller-Wiefel DE, Mehls O and the Mid European Pediatric Peritoneal Dialysis Study Group (MEPPS) (1999) Current practice of peritoneal dialysis in children: results of a longitudinal survey. Perit Dial Int 19(suppl 2):445–449
Furth SL, Donaldson LA, Sullivan EK, Watkins SL for the North American Pediatric Renal Transplant Cooperative Study (2000) Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 15:179–182
Warady BA (1988) Peritoneal dialysis catheter related infections in children. Pediatr Infect Dis J 17:1163–1166
Rinaldi S, Sera F, Verrina E, Edefonti A, Perfumo F, Sorino P, Zacchello G, Andreetta B, Ardissino G et al (1998) The Italian Registry of Pediatric Chronic Peritoneal Dialysis: a ten year experience with chronic peritoneal dialysis catheter. Perit Dial Int 18:71–74
Warady BA, Hebert D, Sullivan EK, Alexander SR, Tejani A (1997) Renal transplantation, chronic dialysis and chronic renal insufficiency in children and adolescents. The 1995 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 11:49–64
Warady BA et al (2000) ISPD guidelines/recommendations. Perit Dial Int 20:610–624
Pierratos A (1984) Peritoneal dialysis glossary. Perit Dial Bull 4:2–3
Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS (1999) Long-term outcome of peritoneal dialysis in infants. J Pediatr 136:24–29
Holley JL, Bernardini J, Piraino B (1994) Infecting organism in continuous ambulatory peritoneal dialysis patients on the Y-set. Am J Kidney Dis 23:569–573
Piraino B (1996) Exit-site care. Perit Dial Int 16(suppl 1):336–339
Honda M (1999). The 1997 report of the Japanese National Registry data on pediatric peritoneal dialysis patients. Perit Dial Int 19(2):473–478
Khanna R, Twardowski ZJ (1988) Peritoneal catheter exit-site (Editorial). Perit Dial Int 10:25–29
Helfrich GB, Winchester JF (1982) Shaving of external cuff of peritoneal catheter. Perit Dial Bull 2:183
Suh H, Wadhwa NK, Cabrala T (1995) Subcutaneous cuff removal in in persistent exit-site tunnel infections in peritoneal dialysis. Adv Perit Dial 11:157–159
Reissman P, Lyass S, Shiloni E, Rivkind A, Berlatzky Y (1998) Placement of a peritoneal dialysis catheter with routine omentectomy–does it prevent obstruction of the catheter? Eur J Surg 164:703–707
Rinaldi S, Sera F, Verrina E, Edefonti A, Gianoglio B, Perfumo F, Sorino P, Zacchello G et al (2004) Chronic peritoneal dialysis catheters in children: a fifteen-year experience of the Italian Registry of Pediatric Chronic Peritoneal Dialysis. Perit Dial Int 24:481–486
Washburn KK, Currier H, Salter KJ, Brandt ML (2004) Surgical technique for peritoneal dialysis catheter placement in the pediatric patient: a North American survey. Adv Perit Dial 20:218–221
Honda M, Litaka K, Kawaguchi H, Sakurak H, Shunji A, Takao K, Tuzuki K et al (1996) The Japanese Registry data on pediatric CAPD patients: a ten-year experience. Perit Dial Int 16:269–275
Stegmair B, Hedberg B, Sandzen B (1990) Absence of leakage by insertion o peritoneal dialysis catheter through the rectal muscle. Perit Dial Int 10:53–55
Leblanc M, Ouimet D, Pichette V (2001) Dialysate leaks in peritoneal dialysis. Semin Dial 14:50–54
Acknowledgments
The authors wish to thank the “Associazione per il Bambino Nefropatico” for its constant and kind collaboration. This study would not have been possible without the important technical and organizational contribution of Mario Scirocco.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macchini, F., Valadè, A., Ardissino, G. et al. Chronic peritoneal dialysis in children: catheter related complications. A single centre experience. Ped Surgery Int 22, 524–528 (2006). https://doi.org/10.1007/s00383-006-1685-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-006-1685-9