Abstract
Purpose
Cerebellar mutism syndrome (CMS) is a severe neurological complication of posterior fossa tumour surgery in children, and postoperative speech impairment (POSI) is the main component. Left-handedness was previously suggested as a strong risk factor for POSI. The aim of this study was to investigate the relationship between handedness and the risk of POSI.
Methods
We prospectively included children (aged < 18 years) undergoing surgery for posterior fossa tumours in 26 European centres. Handedness was assessed pre-operatively and postoperative speech status was categorised as either POSI (mutism or reduced speech) or habitual speech, based on the postoperative clinical assessment. Logistic regression was used in the risk factor analysis of POSI as a dichotomous outcome.
Results
Of the 500 children included, 37 (7%) were excluded from the present analysis due to enrolment at a reoperation; another 213 (43%) due to missing data about surgery (n = 37) and/or handedness (n = 146) and/or postoperative speech status (n = 53). Out of the remaining 250 (50%) patients, 20 (8%) were left-handed and 230 (92%) were right-handed. POSI was observed equally frequently regardless of handedness (5/20 [25%] in left-handed, 61/230 [27%] in right-handed, OR: 1.08 [95% CI: 0.40–3.44], p = 0.882), also when adjusted for tumour histology, location and age.
Conclusion
We found no difference in the risk of POSI associated with handedness. Our data do not support the hypothesis that handedness should be of clinical relevance in the risk assessment of CMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebellar mutism syndrome (CMS) is a possible complication of posterior fossa tumour surgery in children, occurring in approximately 30% of cases [1]. It is characterised by postoperative speech impairment (POSI) usually accompanied by ataxia, emotional lability, hypotonia and brainstem dysfunction, with cranial nerve dysfunction, dysphagia and long tract signs [2].
The pathophysiology of CMS is not yet fully understood, although evidence from imaging studies suggests injury to the dentato-thalamo-cortical pathway (DTCp). The current understanding is that CMS occurs as a result of reduced neural input from the cerebellum to the cerebrum, putatively due to surgical injury to the proximal part of the cerebellar outflow tracts in the DTCp, with associated supratentorial hypoperfusion [3,4,5,6,7].
Well-established risk factors for developing CMS include tumour histology of medulloblastoma, midline tumour location in the cerebellum and brainstem invasion [1, 8,9,10,11].
Left-handers have been shown to be more susceptible to neurological disorders such as migraine, developmental learning disorders and epilepsy [12, 13]. Left-handedness has also been suggested as a strong risk factor for developing CMS, with the hypothesis that left-handed patients are more susceptible to damage to the DTCp, corresponding to an odds ratio of 16.9 (CI: 1.76–847.6) [14].
The aim of this study was to investigate the relationship between handedness and risk of POSI in children included in the Nordic-European study on CMS in children operated for posterior fossa brain tumours (The CMS study) [1].
Methods
The CMS study was designed as a prospective observational multicentre study involving 26 European centres. Between 11th August 2014 and 24th August 2020, we enrolled children aged 0 − 17.9 years undergoing resection or open biopsy of posterior fossa tumours in one of the participating centres, after obtaining informed consent from the parents. The study was approved in Denmark by the Research Ethics Committees of the Capital Region (H-6–2014-002). The design of the study and list of participating centres were published previously [1].
Patient demographics and handedness were recorded as part of the preoperative assessment. In case of emergency surgery, patient enrolment was done within 7 days of surgery. Assessment of handedness was based on the clinical examination and information from the patient and the parents.
Postoperative speech status was the primary outcome, assessed within 2 weeks of surgery as previously published [1]. For the purpose of this study, we considered patients to have postoperative speech impairment (POSI) if they were mute, i.e. no speech production, or if the speech production was severely reduced and limited to single words or short sentences. Speech was considered habitual if the patient did not meet the aforementioned criteria of POSI upon clinical assessment of speech function postoperatively.
Tumour location was recorded by the operating surgeon within 72 h of surgery in a previously published surgical report form [1]. MRI data were not available for this particular study.
Tumour histology was assessed by local pathologists and categorised as pilocytic or pilomyxoid astrocytoma (PA), medulloblastoma (MB), ependymoma (EP), atypical teratoid/rhabdoid tumour (ATRT) or “other tumours”. All data was entered into a secure online database.
Statistical analysis
In the univariate analysis, we used logistical regression to estimate the odds ratio (OR) of postoperative speech impairment dependent on handedness. A possible association between handedness and tumour histology was assessed in a multivariate model. In a second step, we added tumour location, where we considered mutually exclusive categories: brainstem, fourth ventricle without brainstem, vermis without brainstem or fourth ventricle, and finally cerebellar hemispherical without brainstem, fourth ventricle or vermis.
In a sensitivity analysis, we considered POSI as an ordinal outcome with three levels: (1) habitual, (2) reduced speech, and (3) mutism. In a subgroup analysis, we excluded children < 5 years at diagnosis to match the population in the study by Law et al. [14].
Only patients undergoing primary tumour surgery were included in the analyses. Observations with missing values were excluded from all analyses. OR was provided with 95% confidence intervals (95% CI) and p-values. p-values < 0.05 were considered statistically significant. Statistical analyses were performed in R Studio (v. 3.6.2).
Results
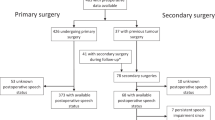
A total of 500 patients were enrolled. We excluded 37 patients (7%) from the analysis due to enrolment at reoperation. Data were missing in another 213 patients (43%) in one or more of three key data categories: surgical data (n = 37), handedness (n = 146), and/or postoperative speech status (n = 53).
The remaining 250 patients (50%) had a median age of 7.6 years (inter-quartile range: 4.9–11.2) and 129 of the patients (52%) were males (Table 1). Left-handedness was observed in 20 patients (8%) and right-handedness in 230 (92%). Among 426 patients undergoing primary surgery and with surgical data, handedness was known in 74% of patients aged 3 years and older (Table 2).
POSI occurred in 66 patients (26%), equally frequently in left- and right-handers when all ages were included (Table 3 [OR for POSI, with left-handedness as reference: 1.08, 95% CI: 0.40–3.44, p = 0.89]). For patients older than 5 years of age, the risk estimate for left-handed children was higher, but not statistically significant (Table 3 [OR: 3.84, 95% CI: 0.72–71.12, p = 0.20]). No significant difference was found when further adjusting for tumour type and location (Fig. 1, Table 3). A sensitivity analysis considering POSI as an ordinal outcome showed a similar result (Table 3).
Absolute risk of POSI with 95% confidence interval in the groups, defined by tumour type and handedness. Hand: handedness; MB: medulloblastoma; Other tumour: all other tumours in one group. Five patients with MB were left-handed; 71 patients with MB were right handed; 14 patients with other tumours were left-handed; 139 patients with other tumours were right handed
Out of the 250 patients in the final analysis, 11 patients underwent open biopsy, two of them left-handed and nine right-handed. A sensitivity analysis excluding these 11 patients reached the same result (OR for POSI, with left-handedness as reference: 1.06 [95% CI: 0.33–2.95]).
Discussion
In our large prospective study, we found no evidence of a statistically significantly increased risk of POSI in left-handed children undergoing tumour surgery of the posterior cranial fossa. To our knowledge, no studies have confirmed that left-handed children are at higher risk of developing CMS since the hypothesis was suggested by Law et al. [14] based on a study including 51 patients undergoing tumour resection in the posterior fossa. This study reported CMS in six out of seven (86%) of left-handers and 11 out of 44 (25%) of right-handers, corresponding to an OR of 16.9 (CI: 1.76–847.6). Surprisingly, all left-handed patients with medulloblastoma (MB) in this study developed CMS. Law et al. considered CMS as “markedly reduced speech output or no speech output” [14], which is similar to the classification of POSI as used in the present study, and as such we consider these two studies comparable [1].
Our data cannot rule out a three times higher risk of POSI (upper 95% CI: 3.44) associated with left-handedness but cannot confirm left-handedness being a strong risk factor for POSI. In fact, it is not possible to statistically exclude any hand preference as a CMS risk factor based on our data. In the present study, we analysed data from 250 children aged 0–17.9 years, whereas Law et al. only included children aged 5 years or older. Excluding patients below 5 years of age from our analysis did not change the risk factor calculations.
Bilateral damage to the superior cerebellar peduncles (SCP), which constitute the proximal part of the DTCp, has been shown to be associated with development of CMS [15,16,17,18,19,20]. Other studies have shown that unilateral SCP damage — either right [14, 21,22,23] or left [24, 25] — is associated with the development of CMS. To our knowledge, no study with diffusion-weighted imaging has yielded substantial results supporting the theory of left-handedness and DTCp damage among children with CMS. Noticeably, studies with sufficient statistical power for addressing this issue can be difficult to complete due to the relatively low prevalence of left-handedness in the general population [26]. Interestingly, Toescu et al. found no difference in radiographic microstructural metrics between right and left DTCp in 30 healthy children of which five were left-handed [27].
The right cerebellum is connected with the left frontocentral cortex via efferent projections in the SCP decussating in the midbrain [27]. Furthermore, the right cerebellum is demonstrated to play an active role in language tasks in the prevailing hemispheric lateralization. For atypical right-hemispheric language dominance, tasks involving language have been suggested to activate the left cerebellar hemisphere, although the actual lateralization on a cerebellar level remains unknown [28]. Data on bilateral hemispheric dominance and cerebellar activity in relation to language tasks has yet to be published. The connected cortical areas include the supplementary motor area (SMA) and pre-SMA, which are engaged in semantic and lexical processing [29]. Lateralization of language was not reported in the abovementioned studies; thus, it is unclear whether their finding of an association between damage to the right DTCp and speech deficits depends on a language lateralization in the left cerebrum. Left-handed children are more likely to have atypical language lateralization (bilateral or right lateralization) than right-handed children, although most of them would have typical lateralization [30]. Accordingly, a study on an adult population (ages 19–46 years) by Knecht et al. found that 4% of strong right-handers, 27% of strong left-handers and 15% of ambidextrous had atypical right hemispheric language dominance [31].
Language lateralization is complex and exhibits significant plasticity [32]. It is likely to be an emergent property of broad brain networks and therefore cannot be inferred from a single afferent white matter pathway. Future studies incorporating a lesion-network mapping approach may yield more definitive results [16].
A key strength of our study is the large number of children included and its prospective, multicentre design. However, the validity of results may be limited by the high proportion of excluded cases (50%) due to missing data regarding postoperative speech status and handedness. Furthermore, the natural distribution of handedness resulting in only 20 patients in our cohort being left-handed limited further subgroup analysis of possible association with tumour histology, location or age. We previously demonstrated that younger children are at higher risk of POSI [1], although we have no reason to believe that this created a selection bias, except for the inability to assess younger children in whom language, speech and hemispheric dominance are not fully developed [1, 33, 34].
Another limitation in the current study is that MRI data were not available for the analysis. Quantitative MRI scans are currently being collated across the 26 participating centres, and results will be published separately. Functional MRI to determine hemisphere lateralization is not currently planned as part of this multicentre study but would definitely be of interest in the context of POSI.
Conclusions
We found no association between the risk of POSI and handedness. Our data do not support the hypothesis that handedness should be of clinical relevance in the risk assessment of CMS.
Further research utilising large functional imaging cohorts of paediatric posterior fossa tumours is required to resolve the persisting uncertainty of lateralization of cerebellar damage in CMS.
References
Gronbaek JK, Wibroe M, Toescu S, Fric R, Thomsen BL, Moller LN, Grillner P, Gustavsson B, Mallucci C, Aquilina K, Fellows GA, Molinari E, Hjort MA, Westerholm-Ormio M, Kiudeliene R, Mudra K, Hauser P, van Baarsen K, Hoving E, Zipfel J, Nysom K, Schmiegelow K, Sehested A, Juhler M, Mathiasen R, group CMSs (2021) Postoperative speech impairment and surgical approach to posterior fossa tumours in children: a prospective European multicentre cohort study. Lancet Child Adolesc Health 5:814–824
Gudrunardottir T, Morgan AT, Lux AL, Walker DA, Walsh KS, Wells EM, Wisoff JH, Juhler M, Schmahmann JD, Keating RF, Catsman-Berrevoets C, Iceland Delphi G (2016) Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst 32:1195–1203
Ahmadian N, van Baarsen KM, Robe P, Hoving EW (2021) Association between cerebral perfusion and paediatric postoperative cerebellar mutism syndrome after posterior fossa surgery-a systematic review. Childs Nerv Syst
Avula S, Mallucci C, Kumar R, Pizer B (2015) Posterior fossa syndrome following brain tumour resection: review of pathophysiology and a new hypothesis on its pathogenesis. Childs Nerv Syst 31:1859–1867
Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K (2011) Cerebellar mutism: review of the literature. Childs Nerv Syst 27:355–363
Patay Z (2015) Postoperative posterior fossa syndrome: unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Childs Nerv Syst 31:1853–1858
Toescu SM, Hettige S, Phipps K, Smith RJP, Haffenden V, Clark C, Hayward R, Mankad K, Aquilina K (2018) Post-operative paediatric cerebellar mutism syndrome: time to move beyond structural MRI. Childs Nerv Syst 34:2249–2257
Reed-Berendt R, Phillips B, Picton S, Chumas P, Warren D, Livingston JH, Hughes E, Morrall MC (2014) Cause and outcome of cerebellar mutism: evidence from a systematic review. Childs Nerv Syst 30:375–385
Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC, Children’s Oncology G (2006) Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg 105:444–451
Catsman-Berrevoets CE, Van Dongen HR, Mulder PG, Paz y Geuze D, Paquier PF, Lequin MH (1999) Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 67:755–757
Catsman-Berrevoets CE, Aarsen FK (2010) The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex 46:933–946
Geschwind N, Behan P (1982) Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci USA 79:5097–5100
Lewin J, Kohen D, Mathew G (1993) Handedness in mental handicap: investigation into populations of Down’s syndrome, epilepsy and autism. Br J Psychiatry 163:674–676
Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Strother D, Fryer C, McConnell D, Hukin J, Kaise C, Wang F, Mabbott DJ (2012) Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol 14:1294–1303
Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D, Boop F, Sanford R, Ness KK, Ogg RJ (2009) Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 132:3087–3095
Albazron FM, Bruss J, Jones RM, Yock TI, Pulsifer MB, Cohen AL, Nopoulos PC, Abrams AN, Sato M, Boes AD (2019) Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 93:e1561–e1571
McEvoy SD, Lee A, Poliakov A, Friedman S, Shaw D, Browd SR, Ellenbogen RG, Ojemann JG, Mac Donald CL (2016) Longitudinal cerebellar diffusion tensor imaging changes in posterior fossa syndrome. Neuroimage Clin 12:582–590
Ojemann JG, Partridge SC, Poliakov AV, Niazi TN, Shaw DW, Ishak GE, Lee A, Browd SR, Geyer JR, Ellenbogen RG (2013) Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst 29:2071–2077
Avula S, Kumar R, Pizer B, Pettorini B, Abernethy L, Garlick D, Mallucci C (2015) Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro Oncol 17:614–622
Miller NG, Reddick WE, Kocak M, Glass JO, Löbel U, Morris B, Gajjar A, Patay Z (2010) Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol 31:288–294
van Baarsen K, Kleinnijenhuis M, Konert T, van Cappellen van Walsum AM, Grotenhuis A (2013) Tractography demonstrates dentate-rubro-thalamic tract disruption in an adult with cerebellar mutism. Cerebellum 12:617–622
Lee S, Na YH, Moon HI, Tae WS, Pyun SB (2017) Neuroanatomical Mechanism of Cerebellar Mutism After Stroke. Ann Rehabil Med 41:1076–1081
Boisgontier J, Fillon L, Rutten C, Saitovitch A, Dufour C, Lemaitre H, Beccaria K, Blauwblomme T, Levy R, Dangouloff-Ros V, Grevent D, Roux CJ, Grill J, Vincon-Leite A, Saidoun L, Bourdeaut F, Zilbovicius M, Boddaert N, Puget S (2021) A CBF decrease in the left supplementary motor areas: new insight into postoperative pediatric cerebellar mutism syndrome using arterial spin labeling perfusion MRI. J Cereb Blood Flow Metab 271678X211031321
Toescu SM, Bruckert L, Jabarkheel R, Yecies D, Zhang M, Clark CA, Mankad K, Aquilina K, Grant GA, Feldman HM, Travis KE, Yeom KW (2022) Spatiotemporal changes in along-tract profilometry of cerebellar peduncles in cerebellar mutism syndrome. Neuroimage Clin 103000. https://doi.org/10.1016/j.nicl.2022.103000. Epub ahead of print. PMID: 35370121.
Vedantam A, Stormes KM, Gadgil N, Kralik SF, Aldave G, Lam SK (2019) Association between postoperative DTI metrics and neurological deficits after posterior fossa tumor resection in children. J Neurosurg Pediatr 1–7
Cavill S, Bryden P (2003) Development of handedness: comparison of questionnaire and performance-based measures of preference. Brain Cogn 53:149–151
Toescu SM, Hales PW, Kaden E, Lacerda LM, Aquilina K, Clark CA (2021) Tractographic and microstructural analysis of the dentato-rubro-thalamo-cortical tracts in children using diffusion MRI. Cereb Cortex 31:2595–2609
Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster AF, Deppe M, Knecht S (2005) Crossed cerebro-cerebellar language dominance. Hum Brain Mapp 24:165–172
Moore-Parks EN, Burns EL, Bazzill R, Levy S, Posada V, Müller RA (2010) An fMRI study of sentence-embedded lexical-semantic decision in children and adults. Brain Lang 114:90–100
Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, Schapiro MB, Plante E, Holland SK (2012) Left-handedness and language lateralization in children. Brain Res 1433:85–97
Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H (2000) Handedness and hemispheric language dominance in healthy humans. Brain 123(Pt 12):2512–2518
Gurunandan K, Arnaez-Telleria J, Carreiras M, Paz-Alonso PM (2020) Converging evidence for differential specialization and plasticity of language systems. J Neurosci 40:9715–9724
Suzuki K, Ando J, Satou N (2009) Genetic effects on infant handedness under spatial constraint conditions. Dev Psychobiol 51:605–615
McIntosh B, Dodd BJ (2008) Two-year-olds’ phonological acquisition: Normative data. Int J Speech Lang Pathol 10:460–469
Funding
This study was funded by The Danish Childhood Cancer Foundation, The Swedish Childhood Cancer Foundation, The Brain Tumour Charity (UK), The Danish Cancer Society, King Christian IX and Queen Louise’s anniversary grant, The Danish Capitol Regions Research Fund, Dagmar Marshall Foundation and Rigshospitalet’s Research Fund in support of oncology purposes and Braintrust (SCOT). All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. This work is part of Childhood Oncology Network Targeting Research, Organization & Life expectancy (CONTROL) and supported by Danish Cancer Society and the Danish Childhood Cancer Foundation. The funding bodies had no role in the design of the study, analyses, interpretation of the data or decision to submit results.
Author information
Authors and Affiliations
Consortia
Contributions
All authors took part in the collection of data. JKG, AFL, ST, RM and MJ contributed to data analysis and interpretation. JKG, AFL and MJ prepared the first draft of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved in Denmark by the Research Ethics Committees of the Capital Region (H-6–2014-002). The study was approved locally in all participating countries.
Conflict of interest
KS reports personal fees from Jazz Pharmaceuticals, Servier, Amgen, and Medscape, and personal fees and grants from Servier, outside the submitted work. KN reports personal fees from Bayer, EUSA Pharma and Y-mAbs, all outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grønbæk, J.K., Laustsen, A.F., Toescu, S. et al. Left-handedness should not be overrated as a risk factor for postoperative speech impairment in children after posterior fossa tumour surgery: a prospective European multicentre study. Childs Nerv Syst 38, 1479–1485 (2022). https://doi.org/10.1007/s00381-022-05567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05567-8