Abstract
The complex Chiari, characterized by abnormal craniocervical bony anatomy in addition to Chiari tonsillar herniation, is a relatively recent addition to the concepts surrounding the Chiari literature. The primary findings of complex Chiari include craniocervical kyphosis and retroflexed odontoid, both of which can be described with radiographic measurements. This manuscript will outline the background literature regarding Chiari craniocervical morphometrics and supply an algorithm for the general management of complex Chiari patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chiari I malformation (CM1) is one of the most commonly treated pathologies in pediatric neurosurgery. The diagnostic criterion for CM1 is cerebellar tonsil position ≥ 5 mm below the foramen magnum on imaging. Using this definition, the prevalence of CM1 among pediatric patients undergoing MRI is estimated to range from 0.8 to 3.7% [1], but only a fraction of these children become symptomatic and require surgical intervention. The simplistic definition of CM1 masks the complex nature of a heterogeneous disease. In a vast majority of symptomatic patients with CM1, posterior fossa decompression (PFD) with or without duraplasty provides effective relief of preoperative clinical symptoms, such as headache or neck pain. Surgery is also highly effective in reversing CM1-associated syringomyelia. However, it is also apparent that a subset of CM1 patients has a more complex presentation with associated skull base morphologic abnormalities. These patients often do not respond to a simple PFD but require occipital–cervical (OC) fusion with or without ventral decompression of the odontoid.
Complex Chiari malformation
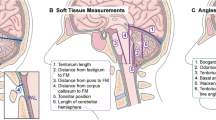
There is not currently a perfect set of parameters to identify the subset of CM1 patients in whom a simple PFD will not be successful. Our group and others have tried over the years to identify morphologic features in CM1 patients that confer a higher risk for the need for repeat surgery and OC fusion. Grabb et al. [2] were the first to report that an increased odontoid retroflexion, defined by a pBC2 distance (maximum perpendicular distance from the basion to the inferoposterior point of the C2 body) > 9 mm, is associated with the need for craniocervical fusion (Fig. 1a). Tubbs et al. [4] made the observation that patients with caudal descent of the brainstem in addition to cerebellar tonsils, termed Chiari 1.5, are at a higher risk for persisting syringomyelia after initial PFD, necessitating repeat surgery (Fig. 1b). The clival–axial angle (CXA) (Fig. 1c) has long been recognized as a cranial–cervical parameter with clinical significance as well [5, 6]. Our group was the first to report a systematic analysis of a series of patients who require OC fusion to identify significant craniovertebral junction metrics associated with need for fusion. The following radiographic parameters were examined: CM1.5, distance of tonsillar descent below the level of the foramen magnum, syringomyelia, medullary kink, basilar invagination, pBC2, and CXA. Our multivariate analysis demonstrated that the significant factors associated with the need for delayed OC fusion are CM1.5, basilar invagination, and CXA < 125° [7].
Illustration of craniocervical parameters. a pBC2 is a line drawn from the inferior portion of the clivus to the posterior-inferior aspect of the C2 vertebral body (arrow). The pBC2 distance (double arrow) is from that line to the posterior superior aspect of the odontoid process (asterisk) of C2. b CXA is the angle (double arrow) derived from a line drawn from the inferior two-thirds of the clivus and a second line drawn from the posterior-inferior C2 body to the superior-posterior aspect of the odontoid. c Basilar invagination is seen when the odontoid process of C2 is above the foramen magnum. McRae’s line is the line joining the basion and the opisthion. The dens should normally be 5 mm below this line (Adopted with permission from Brockmeyer et al. [3])
Clinical decision making
The decision to offer surgical treatment in CM cannot be made based on radiographic findings alone. Integration of clinical symptoms and physical findings with radiographic parameters is crucial in surgical decision making. The most common symptoms attributable to CM are lifestyle-limiting and/or Valsalva-induced suboccipital headaches. Other symptoms, including paresthesia and ataxia, are also often reported. Bulbomyelopathic symptoms such as central sleep apnea, snoring, and dysphagia are more often associated with ventral brain stem compression related to a retroflexed odontoid [3]. Patients younger than 4 years of age can present with symptoms of oral–motor apraxia, such as poor feeding and delayed speech [3, 8]. In the absence of any lifestyle-limiting symptoms, incidentally discovered CM on imaging can generally be monitored without treatment, even in the presence of high-risk radiographic features. The exception is when the patient has a moderate to large syrinx. In our opinion, these patients should be offered surgery because of the otherwise unfavorable long-term outlook [3].
In patients with symptoms referable to CM, the senior author (D.L.B.) uses an algorithmic strategy to identify patients at risk of needing OC fusion, this is based on the currently available evidenced-based literature (Fig. 2) [3]. pBC2 and CXA, which have been shown to have high interobserver reliability [9], are the radiographic parameters primarily used to stratify CM patients with high risks of OC fusion. Patients with a pBC2 > 9 mm or CXA < 125° are considered in the spectrum of “complex Chiari.” In those patients with pBC2 < 9 mm, PFD with or without duraplasty is recommended, but the presence of CXA < 125° increases the overall risk of OC fusion from 1.7 to 13%. Therefore, although CXA < 125° alone does not alter the initial surgical recommendation, it does inform the surgeon and patient that the risk of initial PFD failure is significantly higher than for a patient with a “simple Chiari.” Patients with pBC2 > 9 mm can be further stratified into those with CXA < 125 or ≥ 125°. For those with both high-risk features, pBC2 > 9 mm and CXA < 125°, the need for OC fusion was 83% in Bollo’s paper [7]. However, the majority of those patients underwent primary OC fusions because of severe bulbomyelopathic signs and symptoms. In the senior author’s long-term experience, a much smaller percentage of complex Chiari patients ultimately require fusion after suboccipital decompression and duraplasty. If odontoidectomy is eventually indicated, an endoscopic transnasal/transoral approach is recommended [10]. In our experience, this is well tolerated with minimal surgical morbidity. In patients with pBC2 > 9 mm but CXA ≥ 125°, occipitocervical fusion is infrequently necessary. Although this framework serves as a general guideline for the management of patients with complex Chiari, the final surgical recommendation is made after thorough counseling with patients and their families such that the expectation of the initial surgery and the possible need for further procedures are explicitly discussed.
Treatment paradigm for patients with complex Chairi (Adopted with permission from Brockmeyer et al. [3])
Surgical management
A detailed discussion of the relative merits of various surgical techniques is beyond the scope of this review. However, brief comments on the surgical approaches as they relate to the treatment of complex Chiari are warranted.
PFD, C1 laminectomy, tonsillar shrinkage, and duraplasty (Fig. 3a)
-
The senior author recommends a “T-shaped” opening of the suboccipital fascia and musculature. This facilitates watertight fascial closure at the end and decreases the risk of cerebrospinal fluid (CSF) leakage.
-
Excessive suboccipital bony removal is not recommended, especially in the lateral aspect of the suboccipital bone as it is often the anchoring points for instrumentation in an OC fusion. We generally limit the craniectomy to less than 3 × 3 cm in height and width, respectively. Particular focus is placed on decompressing the lateral foramen magnum to ensure the entire dorsal aspect of the foramen is removed.
-
The fourth ventricular outlet is explored to ensure no arachnoid web or scarring is present to obstruct CSF outflow.
-
Blood is meticulously prevented from entering the intradural space during the entire procedure because intradural blood promotes scarring and arachnoiditis.
-
The authors have used both synthetic dura substitute or pericranial autograft for duraplasty. However, it is our anecdotal experience that pericranial grafts are better tolerated with lower incidence of chemical meningitis or CSF leak.
-
It is important to obtain a watertight CSF closure to minimize the risk of postoperative CSF leak.
Illustration of PFD and OC fusion. a T-shaped incision in the suboccipital fascia and musculature facilitating a watertight closure. b Odontoid reduction, OC fusion. Gray, occipitocervical fusion plates and rods; blue, C2 pars screws; pink, occipital screws (Adopted with permission from Brockmeyer et al. [3])
OC fusion and odontoid reduction (Fig. 3b)
-
The authors generally perform O-C2 instrumented fusion using C2 pars screws anchored to occipital plates bilaterally. A study by Hankinson et al. [11] demonstrated that there is no difference in fusion rate with O-C2 fixation with or without C1 instrumentation using either a C1 lateral mass screw or transarticular screw construct.
-
Manual intraoperative reduction of the odontoid can be performed with gentle distraction and extension of the instrumentation between occiput and C2.
-
Posterior rib autografts are harvested and contoured as graft material for arthrodesis. However, Robinson et al. [12] recently demonstrated no difference in fusion rate between autograft and alternative graft materials in the adult and pediatric populations.
-
Multistranded titanium cables and craniofacial screws are used to anchor the rib autograft against the bony contact surface of the graft with the occipital and C2.
-
Fusion adjunct, such as recombinant human bone morphogenetic protein (rhBMP), is used at the discretion of the surgeon. The senior author uses a small amount of rhBMP at the bony contact points to facilitate fusion.
Transnasal endoscopic odontoid resection
-
This procedure is performed with an otolaryngologist experienced in endoscopy. The endoscope is inserted transnasally to visualize the posterior pharynx overlying the C1 arch and odontoid.
-
The operative instruments are then inserted transorally and transnasally to open the pharyngeal mucosa and drill out the anterior arch of C1 and odontoid. The posterior longitudinal ligament is left intact. An O-arm scan is routinely performed to confirm adequate decompression of the odontoid.
-
The pharyngeal mucosa is closed by the otolaryngologist. The perioperative management is similar to a typical tonsillectomy, where the patient can advance to a semi-solid diet on postoperative day 1.
Summary
CM1 patients have a constellation of symptoms and radiographic findings. Although a simple PFD with or without duraplasty is an effective surgical treatment for most patients, a subset of CM1 patients is at high risk of surgical failure and require OC fusion with or without ventral decompression. At present, these patients are best stratified based on two radiographic parameters, pBC2 > 9 mm and CXA < 125°. Patients with both high-risk features and severe bulbomyelopathy are considered for upfront posterior OC fusion. If clinical symptoms persist after the fusion procedure, endoscopic transoral odontoidectomy is recommended. Patients with pBC2 < 9 mm and CXA < 125° are recommended to have a simple PFD with the understanding that the risk of needing OC fusion is higher than in those with CXA ≥ 125°. For those with pBC2 > 9 but CXA ≥ 125°, the surgical decision is based on the presence of bulbar symptoms. Although this treatment paradigm provides a framework for clinical decision making, it is important to acknowledge that it is based on data from retrospectively collected case series. Further study is needed to confirm the clinical relevance of these measurements in a prospective manner.
References
Martin BA, Geh N, Loth F et al (2017) Morphometric and volumetric comparison of 102 children with symptomatic and asymptomatic Chiari malformation type I. J Neurosurg Pediatr 21:65–71. https://doi.org/10.3171/2017.8.peds17345
Grabb PA, Mapstone TB, Oakes WJ (1999) Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery 44:520–528
Brockmeyer DL, Spader HS (2015) Complex Chiari malformations in children: diagnosis and management. Neurosurg Clin N Am 26:555–560. https://doi.org/10.1016/j.nec.2015.06.002
Tubbs RS, Iskandar BJ, Bartolucci AA, Oakes WJ (2010) A critical analysis of the Chiari 1.5 malformation. J Neurosurg Pediatr 101:179–183. https://doi.org/10.3171/ped.2004.101.2.0179
Nagashima C, Kubota S (1983) Craniocervical abnormalities. Modern diagnosis and a comprehensive surgical approach. Neurosurg Rev 6:187–197
Smoker WR (1994) Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics 14:255–277. https://doi.org/10.1148/radiographics.14.2.8190952
Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL (2012) Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion. J Neurosurg Pediatr 10:134–141. https://doi.org/10.3171/2012.3.peds11340
Albert GW, Menezes AH, Hansen DR, Greenlee JDW, Weinstein SL (2010) Chiari malformation type I in children younger than age 6 years: presentation and surgical outcome. J Neurosurg Pediatr 5:554–561. https://doi.org/10.3171/2010.3.PEDS09489
Martin JE, Bookland M, Moote D, Cebulla C (2017) Standardized method for the measurement of Grabb’s line and clival-canal angle. J Neurosurg Pediatr 20:352–356. https://doi.org/10.3171/2017.5.peds17181
Hankinson TC, Spinks TJ, Grunstein E et al (2010) Transnasal odontoid resection followed by posterior decompression and occipitocervical fusion in children with Chiari malformation type I and ventral brainstem compression. J Neurosurg Pediatr 5:549–553. https://doi.org/10.3171/2010.2.peds09362
Hankinson TC, Avellino AM, Harter D, Jea A, Lew S, Pincus D, Proctor MR, Rodriguez L, Sacco D, Spinks T, Brockmeyer DL, Anderson RCE (2010) Equivalence of fusion rates after rigid internal fixation of the occiput to C-2 with or without C-1 instrumentation. J Neurosurg Pediatr 5:380–384. https://doi.org/10.3171/2009.10.PEDS09296
Robinson LC, Anderson RCE, Brockmeyer DL, Torok MR, Hankinson TC, Pediatric Craniocervical Society (2018) Comparison of fusion rates based on graft material following occipitocervical and atlantoaxial arthrodesis in adults and children. Oper Neurosurg 15:530–537. https://doi.org/10.1093/ons/opy013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of
interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ho, W.S., Brockmeyer, D.L. Complex Chiari malformation: using craniovertebral junction metrics to guide treatment. Childs Nerv Syst 35, 1847–1851 (2019). https://doi.org/10.1007/s00381-019-04214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04214-z