Abstract
Introduction
Benign extracerebral fluid collection (bECFC) can be complicated by subdural hematoma (SDH) or subdural fluid collection (SDFC). The etiology, natural history, and management strategy for SDH/SDFC in bECFC are not fully understood. We retrospectively reviewed the cases of bECFC patients complicated with SDH/SDFC and tried (1) to confirm the fact that bECFC children are vulnerable to SDH/SDFC, (2) to investigate the clinical significance of 'trauma history' witnessed by a caregiver, and (3) to determine optimal management for them.
Method
Among 213 bECFC patients identified from January 2000 to August 2015, 20 patients (male:female = 14:6; median age, 6.5 months; range 1–16 months) complicated by SDH/SDFC documented with brain imaging were evaluated for their clinical manifestations, radiologic features, and management outcomes. The median follow-up period was 9.5 months. They were divided into two groups (traumatic group versus non-traumatic group) according to whether objective radiologic evidence of head injury was present or not, and the two groups were analyzed for any clinical differences between them. We also evaluated the clinical significance of witnessed traumatic events by caregivers as an additional independent variable in the analysis.
Results
The incidence of SDH/SDFC in bECFC patients was 9.4% (20/213) in our data. In a comparative analysis, the traumatic group is more likely to have 'acute' stage SDH, whereas the non-traumatic group is more likely to have 'chronic' stage SDH. The trauma history witnessed by caregivers did not show clinical significance in the data analysis when included as an independent variable. The prognosis of SDH/SDFC in bECFC patients was favorable without surgery in most of patients regardless of whether the patient has evidence of head trauma or not.
Conclusion
Benign ECFC is vulnerable to SDH/SDFC development. For the bECFC patients complicated by SDH/SDFC, the trauma history witnessed by a caregiver did not show any clinical significance. A 'wait and watch' strategy is sufficient for the management of SDH/SDFC in bECFC patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign extracerebral fluid collection (in infancy) (bECFC), also known as external hydrocephalus, is an age-related self-limiting condition characterized by excessive fluid accumulation in the subarachnoid spaces. It usually occurs in infancy, and spontaneous resolution can be expected by approximately 2 to 3 years of age [10]. The term benign is coined because most cases of this condition do not require any neurosurgical intervention, and these children usually achieve normal developmental milestones without clinical sequelae [9]. However, this disease entity is not always benign because subdural hematoma (SDH) or subdural fluid collection (SDFC) can occur as a complication, and the susceptibility to SDH/SDFC in these patients has been reported in the literature [10, 15]. The treatment indication for patients with SDH in bECFC is not yet established. In addition, appropriate management principles are controversial.
Previously, SDH/SDFC in bECFC infants was often thought to be related with child abuse. After better understandings for bECFC patients, however, it is noticed that SDH/SDFC in bECFC is not pathognomonic for child abuse and can occur with minor trauma or spontaneously [8]. On the other side of proposed clinical significance of child abuse, our experience suggested that patients have a low correlation with head trauma history and we checked these patients for analysis of their clinical characteristics.
We retrospectively reviewed cases of bECFC patients with SDH/SDFC diagnosed from our institute and attempted (1) to confirm that bECFC children are vulnerable to SDH/SDFC, (2) to investigate the significance of 'trauma history' witnessed by caregivers in the absence of objective signs of head injury, and (3) to determine optimal management for these patients. Therefore, we analyzed the differences between traumatic patients and non-traumatic patients in their clinical features, radiologic findings, managements, and outcomes. We also reviewed articles and discussed our hypothesis on the mechanism of SDH/SDFC development in bECFC, as a predisposing factor.
Methods
Patients who visited Seoul National University Children’s Hospital between January 2000 and August 2015 were evaluated for inclusion in this study. We enrolled patients with bECFC by searching for diagnoses of 'benign ECFC', 'benign ECFC in infancy', and 'fluid collection' (International Classification of Diseases, tenth revision). Our clinicians made the diagnosis of bECFC when there is a prominent subarachnoid space in the imaging study, including computer tomography (CT), magnetic resonance imaging (MRI), and brain ultrasonography (USG). From our search of clinical data, we identified 213 bECFC patients. Among these 213 cases, after review of the medical records and images, we identified 20 patients (9.4%) who had radiologic evidence of SDH or SDFC associated with bECFC documented in CT (n = 7 patients), MRI (n = 12 patients), or brain USG (n = 1 patient). We excluded patients with hydrocephalus, cerebrospinal fluid shunts, brain atrophy, congenital anomaly, metabolic disease, and coagulopathy. We conducted a comprehensive review of the history and a careful interview and could not find any suspicions of non-accidental traumatic brain injury in our patients (e.g., discrepancy between the traumatic lesion and the history of trauma, presence of associated lesions, lesions of different ages, parental delay in seeking medical attention, or inappropriate and inconsistent reaction of parents). No patient had a history of a dysfunctional family or a suspicion of child abuse.
The medical records of included patients were reviewed retrospectively, and data were collected regarding patient demographics, clinical and radiological features, treatment, and clinical outcomes. The median follow-up period was 9.5 months (range 0–127 months). The overall clinical outcomes (n = 20 patients) were divided into two categories: (1) favorable, where the initial clinical symptoms improved without any sequelae or initial clinical symptoms remained stationary without worsening if the symptoms do not disturb daily activities, and (2) unfavorable, where the disturbing symptoms were not improved, the initial symptoms were aggravated, or neurologic symptoms were newly developed.
Patient images were analyzed for midline shift, location and thickness of the SDH/SDFC, and the stage of SDH/SDFC (acute, subacute, or chronic according to the density in CT or signal intensity in MRI, although it does not always correspond to chronicity in cases of ‘SDFC’). For the patients (n = 17) whose CT or MRI data was available and measurable, we approximated the amount of SDH/SDFC at the imaging slice where the linear length between each corner of the subdural crescent is longest. The amount was estimated by measuring the maximum thickness of hematoma, which was measured from the inner table of the skull (Fig. 1). The follow-up radiological findings were available in 18 patients; they were compared with the initial findings, and the results were classified into two groups: (1) The favorable group included those patients whose follow-up images revealed complete or partial resolution of SDH/SDFC or a stable amount of SDH/SDFC in patients who have no symptoms that disturb daily activities. (2) The unfavorable group included those patients whose symptoms or signs were aggravated, when follow-up images showed radiologically no decrease of SDH/SDFC amount if disturbing symptoms were present, or aggravating signs such as increased amount of SDH/SDFC, increased midline shift, and new active bleeding.
We divided the patients into two groups according to whether they have any identifiable radiologic evidence of head trauma or not, such as scalp contusion or skull fracture. In this first data set, the traumatic group consisted of 7 patients and the non-traumatic group consisted of 13 patients. The patients in this study were designated according to the criteria of the first data set: the traumatic group as 'T' and the non-traumatic group as 'N' (Tables 1, 2, and 3). We compared the two groups to determine any differences in clinical and radiological features between them.
Furthermore, apart from the radiologic objective signs of head trauma, we also wanted to investigate the significance of trauma history witnessed by the patient’s caregiver. When we redefined the traumatic group by including trauma history as an additive inclusion criterion, in this second dataset, the traumatic group consisted of 10 patients and the non-traumatic group included 10 patients; 3 patients (N4, N8, and N11) who did not have radiologic evidence of trauma and previously belonged to the non-traumatic group were reclassified to the traumatic group because of witnessed trauma history. We compared these two reclassified groups in clinical and radiological features.
The data were analyzed by using the software SPSS, version 19 (SPSS Inc., Chicago, IL, USA). Non-parametric continuous values were compared using the Mann-Whitney U test. Differences between categorical variables were analyzed using Fischer’s exact test. Two-sided p values < 0.05 were considered statistically significant.
Result
Incidence of SDH/SDFC among the bECFC patients
Among the 213 patients of bECFC in our data, 20 patients (9.4%) were complicated by SDH/SDFC.
Clinical features and outcomes
We identified 20 patients with bECFC complicated by SDH/SDFC, 14 males and 6 females (M/F ratio = 2.3:1), with a median age at diagnosis of 6.5 months (range 1–16 months). Ten patients were younger than 6 months, the other ten patients were older than 6 months at presentation, and only 1 patient was older than 12 months (16 months) (Tables 1 and 2). The most common positive symptom or sign was macrocrania (head circumference at or above two standard deviations; n = 5, 25%), followed by seizure (n = 2, 10%) after head trauma. Most patients (n = 13, 65%) were asymptomatic and were found incidentally; these asymptomatic patients sought medical attention for the evaluation after an event of head trauma or for increasing head size. Parents did not report any concern regarding developmental problems related to bECFC or SDH/SDFC. There were four patients who underwent open heart surgery for congenital heart disease, but they did not have heart failure, underlying coagulopathy, or any medications evoking bleeding diathesis. One patient was born preterm and suffered from bacterial meningitis, but the infection was controlled without neurological deficit, and there was no clinical relationship when the patient was diagnosed as SDH in bECFC. One patient was found to have a history of germinal matrix hemorrhage in infancy. One patient had achondroplasia. On neurological examination, all 20 patients showed normal features. During the study period, all patients achieved favorable clinical outcomes regardless of the patient group: traumatic or non-traumatic. Regarding two patients (T3 and T5) who had seizure as a manifestation at diagnosis, one patient (T5) was found doing well without antiepileptic medication at the 2-year follow-up, and another patient (T3) was found to be stable without seizures while taking antiepileptic medication at the 2-year follow-up. There was only one patient who was treated surgically at another hospital (N7). All others were managed conservatively without surgery.
Neuroimaging findings at presentation and follow-up
Table 3 shows the details of neuroimaging findings from the study group. All patients had prominent subarachnoid spaces and concomitant SDH/SDFC. SDH/SDFC was bilateral in 11 patients and unilateral in 9. The most common stage of SDH was chronic (n = 16), followed by acute (n = 3) and subacute (n = 1). The median thickness of SDH was 6 mm (n = 17, range 2–14 mm). Scalp contusion was noted in three patients, and skull fracture was noted in six patients, which provide relatively robust evidence of trauma history. Among them, two patients had scalp contusions and skull fractures at the same time. The overall number of patients who presented identifiable radiologic evidence of trauma was 7, and we categorized these seven patients as the traumatic group. The other 13 patients who did not present radiologic evidence of trauma were categorized as the non-traumatic group. None of the patients had brain midline shift caused by SDH/SDFC.
Among the ten patients (T1–7, N4, N8, N11) who had trauma history, four patients took imaging studies within a day after the accident. Two patients took imaging studies in a delayed fashion (30 and 90 days, respectively, after the accident). The other four patients could not recall the exact date of the accident, but they were found to take imaging studies within 90 days after the trauma.
Follow-up imaging was available in 18 patients, and the average interval between the initial diagnostic imaging and the follow-up imaging was 2 months (median, range 1 day–8 months). All the sequential neuroimaging of the patients demonstrated radiologically favorable results, in which 2 patients showed complete resolution of SDH/SDFC, 11 showed marked reduction, and 5 showed no change. No recurrence of SDH/SDFC had been encountered during the study period.
Comparison between traumatic and non-traumatic patients
The differences in demographics, clinical features, radiologic findings, and clinical outcomes between the two groups of the first data set were investigated (Table 4). There were two patients who experienced seizures as a presenting sign in the traumatic group, but the difference was not statistically significant. Concerning the stage of SDH, only the traumatic group had three patients with acute hematoma, and the non-traumatic group had no patients with acute hematoma (p = 0.031). Chronic stage SDH tends to occur more in the non-traumatic group than the traumatic group (p = 0.007).
As mentioned above, in the second data set, we also investigated the differences between the two groups reclassified according to radiologic evidence of head trauma plus the trauma history as an inclusion criterion (Table 4). In this analysis, there were no statistically significant differences between the two groups. Even concerning the stage of SDH, there was no statistical significance.
Case illustrations
The following cases represent typical courses of different clinical settings: case 1 (T5) for a patient of the traumatic group, case 2 (N5) for a patient of the non-traumatic group, and case 3 (N7) for a patient who had undergone potentially unnecessary drainage surgery for SDH.
Case 1 (T5) (traumatic group)
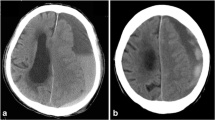
A 7-month-old male infant who had undergone heart surgery for tetralogy of Fallot in the same month was referred to the neurosurgical department for acute SDH documented by a CT scan, which was obtained after the patient’s seizure event. The patient had no signs of an enlarging head circumference and no obvious history of head trauma. The patient had no bleeding tendency and was not taking anticoagulants. The CT scan showed SDH along the cerebral falx, left occipital convexity, and a suggestive sign of occipital bone fracture (Fig. 2a). Together with SDH, there was a radiological feature suggestive of bECFC (Fig. 2b). Even though there was no identified history of head trauma, we assumed an unnoticed head trauma because the patient’s CT scan showed characteristic features of traumatic brain hemorrhage after injury. The patient was neurologically stable, and we recommended imaging follow-up. Follow-up MR images obtained 1 week after the initial image showed a partially resolved state of SDH, and the prominent subarachnoid space was more apparent than in the previous CT scan (Fig. 2c). The 4-month follow-up CT scan showed near-complete resolution of SDH and a concomitant decrease in the subarachnoid space, which is in accordance with the typical course of bECFC (Fig. 2d). This patient achieved normal development without neurological deficits and was doing well at the 2-year follow-up.
Radiological findings of case 1. a A CT scan showing subdural hematoma (SDH) along the cerebral falx and the left occipital convexity (white arrows) and a suggestive sign of occipital bone fracture (white arrowhead). b A CT image showing prominent subarachnoid space in the frontal convexity and anterior interhemispheric fissure (white arrowhead). c MRI taken after 1 week showing subacute SDH at the same site (white arrows). There were enlarged subarachnoid spaces and prominent vascular markings at the bilateral frontal area, which suggest underlying extracerebral fluid collection (white arrowhead). d A follow-up CT scan taken 4 months later showing near-complete resolution of SDH and concomitant decreased subarachnoid space in the bifrontal area
Case 2 (N5) (non-traumatic group)
A 4-month-old male infant was presented with an enlarged head circumference (46.5 cm, > 2 SD). MR images taken at age 3 months revealed an enlarged subarachnoid space and bilateral frontal convexity chronic-stage SDH (Fig. 3a, b). There was no radiological evidence of trauma. The patient had no specific past medical history and no trauma history. The patient’s grandfather also had a large head, suggesting a familial tendency. Because the patient had no neurological symptoms, we recommended conservative management and image follow-up. Brain ultrasonographic images obtained at 7 months of age showed complete resolution of the SDH, and the infant did not reveal any symptoms. Follow-up MR images obtained at 3 years of age showed no signs of SDFC and complete resolution of the bECFC (Fig. 3c). He had achieved all developmental milestones and was doing well at the 3-year follow-up.
Radiological findings of case 2. a MRI showing the enlarged subarachnoid space (white arrowheads) and concomitant bilateral frontal convexity chronic subdural hematoma (SDH) with low signal intensity in a T1-weighted image (white arrows). b T2-weighted MRI showing prominent subarachnoid space and vascular markings within (white arrowhead) and bifrontal chronic SDH (white arrows). c Follow-up T2-weighted MRI taken at 3 years of age showing complete resolution of SDH and extracerebral fluid collection
Case 3 (N7) (a surgical case)
This female patient was born as a preterm infant after 34 weeks of gestation and had been managed for respiratory distress syndrome and bacterial meningitis at a local hospital at 1 month of age. At age 5 months, MR images taken for a routine check-up revealed SDH at the right frontal convexity in association with a prominent subarachnoid space (Fig. 4a). She underwent subdural drainage for approximately 10 days at the local hospital. The patient was referred to our clinic for further management of SDH. The caregiver said that the patient underwent surgery despite the absence of specific symptoms. After drainage, the follow-up CT showed decreased SDH but the amount of SDH plus SDFC was similar (Fig. 4b). The patient had no adverse symptoms. We recommended removal of the drainage catheter and conservative management. The patient developed normally and was doing well at the 3-year follow-up telephone interview.
Radiological findings of case 3. a The initial T1-weighted MRI showing enlarged bifrontal subarachnoid spaces (white arrowheads) and concomitant right frontal convexity subdural hematoma (SDH) (white arrow). b Follow-up T1-weighted MRI obtained 10 days post-operatively revealed decreased SDH (white arrow) and increased subarachnoid space (white arrowheads) with a seemingly unchanged volume of SDH plus extracerebral fluid collection
Discussion
This retrospective review of bECFC patients demonstrates that bECFC is vulnerable to SDH/SDFC development when considering the higher incidence of SDH/SDFC development compared with that of a normal population. We identified 20 bECFC patients complicated by SDH/SDFC and divided them into two groups according to whether there is identifiable radiological evidence of head trauma or not. In the analysis, the traumatic group is more likely to have acute-stage SDH, whereas the non-traumatic group is more likely to have chronic-stage SDH. The trauma history witnessed by caregivers did not show clinical significance in our data analysis when included as an independent variable. Regardless of whether the patient has evidence of head trauma or not, we concluded that SDH/SDFC complicated in the setting of bECFC is self-limited and has a good prognosis. Thus, a “wait and watch” strategy is likely adequate for the management of SDH/SDFC in bECFC patients.
Incidence and mechanism of SDH development in bECFC patients
Despite the benign nature of bECFC, some patients show coincidence of SDH/SDFC, which is found fortuitously or during diagnostic evaluations for trauma. A literature review found that the incidence of SDH/SDFC in bECFC patients was 4–18%, which is similar to the 9.4% incidence in our study [1, 4, 10, 16]. Generally, in the population at large, the incidence of SDH/SDFC in infancy is between 12 and 25 cases per 100,000 children, and most detected cases are revealed during the investigation of physical abuse [5].
Although we raised the suspicion of child abuse in all 20 cases, we examined the fundus only in four patients out of seven who showed radiologic evidence of trauma. If radiologic evidence of trauma or a clue of child abuse was absent, we did not perform routine eye examination. However, routine funduscopy may be a safe and sure way to exclude the possibility of child abuse and should be considered in all patients of bECFC associated with SDH/SDFC.
The correlation between SDH/SDFC and bECFC has already been reported by several other studies, and bECFC has been suggested to be a risk factor for SDH/SDFC development [6, 7, 11]. SDH/SDFC can occur with minor trauma or spontaneously in the setting of bECFC [8, 14]. To explain this phenomenon, there are some proposed mechanisms of SDH/SDFC development in bECFC patients. Bode et al. suggested that bECFC induces craniocerebral disproportion and predisposes patients to the development of SDH or effusion due to brain displacement and subsequent tearing of the bridging vein by head injury [2]. Vinchon et al. reported that bECFC patients are at greater risk of developing SDH because of a fragile hydrodynamic balance induced by the enlarged subarachnoid space [15]. Papasian et al. suggested a mathematical modeling of benign external hydrocephalus that predicts a predisposition towards extra-axial hemorrhage after minor head trauma by increased frequency of venous stretch injury in a widened extra-axial space [13].
We agree with the hypothesis formulated by Papasian that enlarged subarachnoid spaces and the secondary stretching of the bridging veins may predispose patients to the development of SDH, even with minimal or no trauma. In addition to previously suggested mechanisms, we postulate, in keeping with the results of our studies, that enlarged subarachnoid spaces in bECFC produce the intracranial condition called “easily mobile brain” (Fig. 5). In this condition, the brain is easily shifted and the bridging vein is more frequently stretched and injured by minor head trauma. However, because there is plenty of space between the skull and the brain in an enlarged subarachnoid space, there would be a buffering effect, alleviating the impact of head trauma. As a result, even though there is a head trauma and subsequent development of SDH, the brain itself is relatively shielded from damage.
A theoretical model of the 'easily mobile brain' in a benign extracerebral fluid collection (bECFC) patient. a A model of a normal size of subarachnoid space (SAS) without bECFC. b When the patient suffers head trauma, brain displacement usually occurs in the relatively opposite direction of the impact exerted by the trauma. Because the distance between the skull and brain is short in this case, the brain could crash into the skull and become directly damaged. c Enlarged size of SAS in a bECFC patient. In this case, bridging veins are more frequently stretched. d When the bECFC patient suffers head trauma, brain displacement is relatively easier and greater than that of the normal patient because of a greater SAS where the brain can become mobilized. Due to the buffering effect of an enlarged SAS, the brain does not directly crash into the skull and is less likely to be damaged from external impact. On the other hand, because the bridging veins are more prone to stretching and are torn by exacerbated brain movement, subdural hematoma or subdural fluid collection with an accompanying tear of the arachnoid membrane is more likely to occur
It is important to differentiate bECFC which was complicated by SDH/SDFC from fluid collection associated with SDH, especially in chronic SDH. The features of bECFC are as follows: (1) The fluid collection is in the subarachnoid space, not in the subdural space; (2) the density or signal intensity of fluid collection is the same as the cerebrospinal fluid whereas the density or signal intensity of fluid collection as a part of chronic SDH is mostly different from that of cerebrospinal fluid; (3) the fluid collection does not have fluid-fluid level inside; and (4) the fluid collection is always at the anterior part of the brain even in cases where the SDHs are located elsewhere. If the bECFC and SDH/SDFC are simultaneously found, it seems reasonable that the bECFC is a preceding lesion in this age group, especially where recent trauma is associated. It is hard to imagine that the focal SDH/SDFC causes bilateral symmetric enlargement of subarachnoid space.
Differences between the traumatic and non-traumatic groups
When comparing the two groups of the first data set classified according to radiologic evidence of head trauma (scalp contusions and skull fractures), it is more likely that the traumatic group will have acute-stage SDH, and the non-traumatic group will have chronic-stage SDH (Table 4). However, when interpreting the results of this analysis, caution is required regarding the following two issues: First, the 'stage' of SDH in bECFC patients, which can be estimated by CT density and MR signal intensity, may not always correlate with the actual age of the hematoma. Rather, there is a probability that the CT density or MR signal intensity of SDH is determined by the amount of bleeding, which is formed by head trauma and mixed into 'preformed' or 'simultaneous' SDFC (which developed before or simultaneously with the head trauma). From this point of view, we can explain that the small amount of acute bleeding can appear as chronic-stage SDH because its effect may be offset by the relatively larger amount of preexisting or simultaneous SDFC. Second, we should consider the assumption that radiologic evidence (inclusion criteria) itself could imply the existence of a more significant head injury from traumatic events. In this regard, the traumatic group may have more cases of acute-stage SDH because it may convey more significant head damage.
Comparing the second data set, reclassified by adding trauma history as an inclusion criterion, there was no statistically significant difference between the two groups, including the stage of SDH (Table 4). We suggest a potential explanation for this different result from the first data set. The analysis of the second data set is different from the original one in that it includes not only the objective radiologic signs of head trauma but also the trauma history, which mainly comprises the 'witnessed traumatic events' by their guardians. The witnessed traumatic events are a more subjective parameter than the radiologic findings, and this subjectivity may result in low reliability because traumas may be missed or even over-estimated by their guardians, and the severity of impact is hard to estimate. Moreover, because a considerable number of patients could not declare the exact date of the accident and as a result could not identify the exact time interval between the trauma and imaging study, it could be inappropriate to analyze the hematoma age which is usually a time-dependent phenomenon. If we think that the occurrence of SDH/SDFC in bECFC is not correlated with trauma history, the usual lack of its correlation with child abuse is easily understood.
Considering that trauma history has low predictive reliability in this age group and shows less statistical significance than radiologic findings, it would be reasonable to place less emphasis on trauma history when assessing the severity of a patient’s clinical condition. We assume that objective radiologic evidence of head trauma should be the primary consideration for evaluation of the significance of a head injury. However, in our study, whether there is radiologic evidence of trauma or a witnessed traumatic event, all patients showed good clinical outcomes.
Management of SDH in bECFC patients and its prognosis
Because bECFC is regarded as a self-limiting entity, there is a consensus that intervention is not required [12, 13]. However, in cases where bECFC is combined with SDFC, the indication for treatment is controversial.
Nishimura et al. reported the natural history of 20 patients diagnosed with subarachnoid fluid collection. Three patients were complicated by SDH and underwent surgical intervention. The authors insisted that surgical treatment should be considered when subarachnoid fluid collection is complicated by a sizable or symptomatic SDH [11]. There are several reported cases where surgical intervention was performed for SDH complications in bECFC patients [3, 4, 8, 10, 14].
On the other hand, there have also been reports that SDH associated with bECFC can decrease without surgical treatment [4, 8, 14, 16]. Ravid et al. insisted that the prognosis of patients with SDH associated with bECFC is usually better than that of other patients without bECFC [14].
After a literature review of surgical cases of SDH in bECFC, we noticed that many patients underwent surgical intervention despite a lack of significant neurological signs and symptoms. In our study, one patient underwent surgical drainage of SDH at the local hospital despite having no significant neurological symptoms (case 3). Without an understanding of the natural history of this disease entity, there may be unnecessary interventions leading to complications.
To answer the question whether surgery is a justified risk for these patients, we focused on the natural history of SDH/SDFC in bECFC patients. From the review of our patients, we agree that SDH/SDFC in bECFC patients generally presents a good prognosis, and a “wait and watch” strategy is adequate for management of cases without the presentation of significant neurological symptoms. We recommend a 3-month follow-up imaging study, and we usually use brain ultrasonography if it is feasible. Generally, checking a follow-up imaging study and outpatient clinic follow-up interviews were enough for evaluation.
Given the natural history of this disease, the decision of surgical versus conservative management should be made based on the clinical manifestations and the patient’s neurological status. When there are neurological deficits present, including altered level of consciousness, signs of increased intracranial pressure, or concerns regarding potential harmful effects related to SDH/SDFC, therapeutic drainage should be considered. However, it seems relatively rare that SDH/SDFC in bECFC expresses critical clinical symptoms.
Limitations
First, the present study is a retrospective analysis of 20 patients, and the small population may have resulted in a lower statistical power. Second, the follow-up period (median, 9.5 months; range, 0–127 months) may not be sufficient to draw definite conclusions. Although symptomatic and radiological resolutions were found, long-term developmental outcomes were not observed. However, considering previous reports, as well as some of our patients with long-term follow-up, developmental delay following conservative management is probably unlikely. A long-term follow-up prospective study with a larger number of patients will be necessary to establish a more accurate clinical prognosis.
Conclusion
Benign ECFC is vulnerable to SDH/SDFC development. For bECFC patients complicated by SDH/SDFC, the trauma history witnessed by caregivers does not significantly affect clinical features and management decisions. SDH/SDFC in bECFC is a self-limiting condition in which close observation with imaging follow-up would be adequate for successful management.
References
Azais M, Echenne B (1992) Idiopathic pericerebral swelling (external hydrocephalus) of infants. Ann Pediatr (Paris) 39:550–558
Bode H, Strassburg HM (1987) Craniocerebral dysproportion—a contribution to the significance of extracerebral fluid collections in infancy. Klin Padiatr 199:399–402
Ghosh PS, Ghosh D (2011) Subdural hematoma in infants without accidental or nonaccidental injury: benign external hydrocephalus, a risk factor. Clin Pediatr 50:897–903
Hellbusch LC (2007) Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J Neurosurg 107:119–125
Hobbs C, Childs AM, Wynne J, Livingston J, Seal A (2005) Subdural haematoma and effusion in infancy: an epidemiological study. Arch Dis Child 90:952–955
Ikeda A, Sato O, Tsugane R, Shibuya N, Yamamoto I, Shimoda M (1987) Infantile acute subdural hematoma. Childs Nerv Syst 3:19–22
Kapila A, Trice J, Spies WG, Siegel BA, Gado MH (1982) Enlarged cerebrospinal fluid spaces in infants with subdural hematomas. Radiology 142:669–672
McNeely PD, Atkinson JD, Saigal G, O'Gorman AM, Farmer JP (2006) Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. Am J Neuroradiol 27:1725–1728
Ment LR, Duncan CC, Geehr R (1981) Benign enlargement of the subarachnoid spaces in the infant. J Neurosurg 54:504–508
Mori K, Sakamoto T, Nishimura K, Fujiwara K (1993) Subarachnoid fluid collection in infants complicated by subdural hematoma. Childs Nerv Syst 9:282–284
Nishimura K, Mori K, Sakamoto T, Fujiwara K (1996) Management of subarachnoid fluid collection in infants based on a long-term follow-up study. Acta Neurochir 138:179–184
Odita JC (1992) The widened frontal subarachnoid space—a CT comparative-study between macrocephalic, microcephalic, and normocephalic infants and children. Child Nerv Syst 8:36–39
Papasian NC, Frim DM (2000) A theoretical model of benign external hydrocephalus that predicts a predisposition towards extra-axial hemorrhage after minor head trauma. Pediatr Neurosurg 33:188–193
Ravid S, Maytal J (2003) External hydrocephalus: a probable cause for subdural hematoma in infancy. Pediatr Neurol 28:139–141
Vinchon M (2010) Subdural hematoma in infants: can it occur spontaneously? Data from a prospective series and critical review of the literature. Reply. Childs Nerv Syst 26:1485–1485
Yew AY, Maher CO, Muraszko KM, Garton HJ (2011) Long-term health status in benign external hydrocephalus. Pediatr Neurosurg 47:1–6
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2015R1D1A1A01059605).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(XLSX 527 kb)
Rights and permissions
About this article
Cite this article
Lee, H.C., Chong, S., Lee, J.Y. et al. Benign extracerebral fluid collection complicated by subdural hematoma and fluid collection: clinical characteristics and management. Childs Nerv Syst 34, 235–245 (2018). https://doi.org/10.1007/s00381-017-3583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3583-y