Abstract
Background
Cranial fasciitis (CF) is an uncommon benign primary lesion of the skull that typically affects the pediatric age group. Due to the rarity of CF, no prospective studies exist. Earliest description of this condition dates to 1980. The limited scientific and clinical literature regarding CF is dominated by case reports. For these reasons, questions pertaining to the true incidence, genetic risk factors, prognosis, and long-term outcome remain unanswered.
Discussion
Clinically, CF presents as a firm, painless, growing scalp mass that is typically not considered in the differential diagnosis. Preoperative pathognomonic signs and symptoms are absent, and imaging features are often nonspecific. Treatment is typically through complete surgical resection, at which time histopathological examination confirms the diagnosis of CF. Reconstruction of the skull defect in the child is critical. Autograft techniques help maintain a rigid construct that integrates with the native skull while preserving its continued ability to grow. Generally, a good outcome is observed with complete resection.
Exemplary case
We report a case of CF in an infant with emphasis on operative nuances and early follow-up results.
Conclusion
CF is a rare fibroproliferative disease that has a poorly defined incidence and long-term follow-up. Due to its locally invasive nature and nonspecific presentation, CF is often difficult to differentiate from malignancies and infections. Complete surgical resection is the best approach for diagnosis and cure. Its occult clinical presentation often allows it to achieve considerable growth, leaving a sizeable skull defect following resection. Since CF presents in the pediatric population, allograft reconstruction is preferred over titanium mesh or other synthetic materials to allow osseous integration and continued uninterrupted skull growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and historical background
Cranial fasciitis (CF) is a rare, benign fibroproliferative lesion of the skull that typically presents in young children as a firm, painless, growing scalp mass. Earliest description of CF dates to 1980 in a report by Lauer et al. [32]. Radiographically, it often presents as a soft tissue lytic mass eroding through the skull. It may appear isointense on T1 magnetic resonance imaging (MRI) with peripheral contrast enhancement and demonstrates a hyperintense core and an isointense periphery on T2 MRI [27]. This typical radiographic description is not always encountered [57], making the initial diagnosis challenging. CF can present with ambiguous radiographic findings that can mimic neoplastic and infectious conditions [59]. CF commonly occurs spontaneously, but may also arise following trauma [27] or whole-brain radiation [8, 36, 60]. While intralesional steroid injection has been described in a patient had no bone involvement [34], complete surgical resection provides a definitive diagnosis and cure.
To further investigate this entity, we conducted a PubMed literature search for all CF cases and were able to identify only 54 articles [1,2,3,4,5,6,7,8,9, 12,13,14,15,16,17,18, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34, 36,37,38,39,40,41,42, 44,45,46, 48,49,50,51,52,53,54,55, 57,58,59,60,61]. The majority of those articles were case reports (Table 1). A total number of 76 patients from 1980 to 2016 were reported in the literature that we reviewed. Average age of all the patients was 6 years, with a range of 1 month to 61 years. Male to female ratio was approximately 2 to 1 (Fig. 1). The most common site of CF lesion was the temporal region (26%), followed by the parietal (20%), occipital (14%), and frontal (13%) (Fig. 1). There are no large studies describing CF’s natural history, incidence, recurrence rates, or long-term follow-up after treatment. In this paper, we conduct a case-based review of CF and discuss a clinical case that was treated with complete resection with a focus on the technical intraoperative nuances and follow-up.
Clinical presentation
CF is a commonly misdiagnosed primary skull mass that can mimic conditions such as malignancies and infection (e.g., dermoid, epidermoid, Langerhans cell histiocytosis, tuberculosis). Due to its rare occurrence, it is not usually considered in the differential diagnosis of primary skull lesions. No clear natural history was present in the literature; however, several reports have shown that the disease can progress to a significant size and cause brain compression [2, 17, 42, 49, 55].
For multiple reasons, delayed diagnosis of CF is common. Because CF is painless, children often ignore it until it becomes relatively large and noticeable to the parents. Furthermore, because the growth rate is slow, the lesion may grow to a substantial size, over a significant amount of time, prior to causing any neurological symptoms.
Diagnosis
The imaging characteristics of CF have been described as isointense on T1 MRI with peripheral contrast enhancement. On T2 MRI, CF demonstrates a hyperintense core and an isointense periphery on T2 MRI [27]. The imaging characteristics noted in our case did not match the proposed description in the literature. The lesion in our case was diffusely isointense on T1 and T2 MRI. It also demonstrated diffuse postcontrast enhancement on T1-weighted sequences. This mismatch between the reported classic imaging characteristics [27] was also noticed in other reports [57], corroborating the impression that there are no reliable radiologic imaging findings that are distinctly diagnostic for CF.
Management
Surgical resection remains the definitive way to diagnose and treat this condition. The planning phase of surgery begins with patient positioning. The lateral decubitus position allows simultaneous access to a rib bone donor site and the lesion. If the location of the lesion is in the occipital area, then the prone position may be a preferred position to access the donor rib. The periosteum should be carefully preserved and gently elevated from the bone while keeping in mind the location of the neurovascular bundle below the inferior border of the rib. In our experience, once the periosteum is preserved and sutured back, the rib will regrow within 4–12 months (Fig. 2e, f). Depending on the surgeon’s preference, the rib may be morselized or eburnated and plated to the healthy skull edges. The advantage of plating large pieces of eburnated rib is that it provides a strong scaffold when compared to morselized bone.
a, b Preoperative anteroposterior and lateral skull radiographs demonstrating a right parietal lytic lesion with sclerotic border. c, d Postoperative anteroposterior and lateral skull radiographs demonstrating resection of the mass and rib allograft cranioplasty. e Immediate postoperative chest radiograph showing absent eighth rib on the right side (arrows depict the location of the harvested rib). f Four-month follow-up X-rays showing complete regeneration of the rib
During the lesionectomy procedure, it is important to keep in mind that the dura mater has osteogenic capability and therefore should be preserved whenever possible [19, 43]. Involved segments of the dura should be carefully inspected, excised, and repaired appropriately using synthetic dura or fascial grafts. In some instances, the outer dural leaflet can be split under the operative microscope. If the inner dural leaflet is not involved, it should be preserved. In such case, no further dural repair is necessary.
Large skull defects in infants can be reconstructed with harvested bone from rib or split calvarial bone grafts. Unlike adults, where continued skull growth is not a consideration, cranial defects in very young children are preferably not corrected with synthetic implants such as titanium mesh, polyether ether ketone (PEEK), or polymethyl methacrylate (PMMA) when possible. Studies have shown that shortly after birth, the human skull grows rapidly, reaching 90 % of its final size by the age of 7 years, and slows down after puberty [10, 35]. Therefore, in this age group, autograft is usually a good biocompatible option to reconstruct the skull and preserve its ability to grow unconstrained [11, 47, 56]. During lesionectomy, valuable bone dust should be carefully collected and used at the end of the reconstruction procedure.
Similar to the skull, rib grafts provide a tripartite structure consisting of a rigid inner and outer cortical bone separated by a softer inner cancellous bone. The rib’s natural curvature makes it easy to incorporate into large skull defects while maintaining a low-profile construct level with the skull surface (Fig. 2c, d). The ability of ribs to regenerate makes them excellent autologous donor bone candidates. Disadvantages include performing an extra incision, postoperative pain, injury to the neurovascular bundle, and potential for pulmonary complications such as pneumothorax. Split calvarial bone grafts are also excellent candidates to reconstruct large skull defects. Their round and malleable profile provide the ideal contour for the skull. Split calvarial grafts help avoid the morbidity associated with extracranial bone harvesting at the expense of larger cranial incisions to incorporate a large surface area of intact skull. This makes them ideal for large craniosynostosis operations to a greater extent than smaller lesionectomy procedures in infants. In summary, we believe that rib and split thickness calvarial grafts are equivalent and choosing one over the other ultimately depends on the surgeon’s preference.
Prognosis and outcome
There are many questions pertaining to CF that remain unanswered. Further larger studies can aid in elucidating the true incidence, genetic risk factors, prognosis, and long-term outcome of this rare condition.
Exemplary case presentation
Clinical history and examination
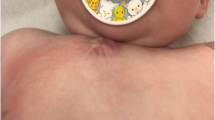
A 6-month-old boy presented to a local physician with a slowly growing painless parietal scalp mass. The child was born full term via a nontraumatic normal vaginal delivery after an uncomplicated intrauterine course. The child had no history of head trauma. Neurologically, the child was normal. Head examination revealed a right parietal firm immobile lesion measuring approximately 3 cm in largest diameter. Overlying skin and scalp hair appeared normal.
Imaging
Skull radiographs demonstrated a parietal lytic lesion a few centimeters lateral to the midline with sharp sclerotic borders (Fig. 2a, b). Computed tomography (CT) scan of the head showed a right parietal soft tissue abnormality measuring 3 cm and eroding through the inner and outer tables of the skull (Fig. 3). MRI redemonstrated the soft tissue lesion previously described on CT. The lesion was isointense on T1 and T2 MRI and enhanced with administration of intravenous gadolinium (Fig. 4).
Preoperative computed tomography (CT) imaging. a Compiled axial slices 1.5 mm in thickness apart showing the lytic lesion (cranial fasciitis) involving the inner and outer tables of the parietal bone with associated overlying soft tissue swelling. b Three-dimensional volume rendering of the CT scan using Slicer 4.6.2 software
Operative technique
Positioning
The patient was positioned in the left lateral decubitus position to allow simultaneous lesionectomy and rib harvesting. The head was rested on a cerebellar headrest. An inverted U-shaped incision was outlined to incorporate the palpable lesion. A diagonal straight incision overlying the ninth rib was outlined (Fig. 5).
Lesion resection
Scalp incision was made as outlined (Fig. 5a, b). The periosteum was then incised and reflected along with the scalp flap inferiorly. A portion of the periosteum that was partially adherent to the lesion was excised and sent for frozen section, which showed a spindle cell proliferation. A grayish tumor eroding through the outer cortical bone was identified (Fig. 5b). A high-speed electrical drill was used to fashion a small craniotomy around the tumor maintaining a grossly clean margin of 5 mm. All of the bone dust was saved for later use. The outer layer of the dura was removed with the skull mass and sent en bloc for histopathological examination. The inner layer of the dura appeared to be grossly free of abnormal tissue, and bipolar cautery was utilized to achieve hemostasis.
Rib harvesting
The chest incision was made with a no. 15 blade scalpel parallel to the underlying eighth rib. Blunt dissection was performed to spread the muscle layers, and bipolar cautery was used to dissect down through the muscle attachments to the ribs. The periosteum of the eighth rib was then incised sharply, and straight curettes and no. 1 Penfield instrument were used to dissect the periosteum over and around the rib. A semi-sharp was then used to complete circumferential dissection of the periosteum off the surface of the exposed rib. A rib cutter was then used to incise 6 cm of rib (Fig. 6). A Valsalva maneuver was performed and no air leak was noted. A Marcaine nerve block was infiltrated proximally at the T7, T8, and T9 ribs for postoperative pain control. The periosteum was reapproximated with absorbable sutures. The muscle fascia and the dermis were closed in layers. The harvested rib was set aside for later use.
Schematic of rib resection technique. After exposing the desired rib (a), the periosteum is incised sharply and the underlying bone is exposed (b). The periosteum is reflected off the bone using a Penfield no. 1 instrument and straight curettes (c). A bone cutter is introduced after circumferential dissection of the periosteum away from the cortical surface of the rib (d)
Cranial reconstruction and autologous rib cranioplasty
The harvested rib was eburnated using an electric drill. It was then cut in half and plated over the defect using a tissue-compatible absorbable osteosynthesis plating system consisting of amorphous pure poly(d,l-lactide) acid (PDLLA) polymer (Fig. 5c). The construct was solid. Approximately 3.5 cm3 of bone putty containing demineralized bone matrix and bone chips combined with the harvested bone dust was placed in the empty spaces (Fig. 5b).
Pathology
Histopathologic examination of the lesion demonstrated a fairly uniform lesion composed of loosely arranged, broad fascicles of spindle cells. The background was variably edematous/myxoid with focal areas of increased collagen. The spindle cells showed minimal nuclear atypia; however, mitotic activity was present. The spindle cell proliferation extensively involves the skull bone (Fig. 7a, b). Immunohistochemical staining showed the cells to express vimentin and smooth muscle-specific actin, while the cells were negative for desmin, muscle-specific actin, and CD34. The morphologic features and immunophenotype in this clinical setting are characteristics for cranial fasciitis.
Clinical outcome
The patient tolerated the surgery well with no complications. Operative time was 2 h and 15 min. Estimated blood loss was 5 ml. A plain chest X-ray (CXR) performed postoperatively did not demonstrate any pneumothorax and showed the area of rib resection (Fig. 2e). Postoperative radiographs at the time showed stable skull construct (Fig. 2c, d). Follow-up CXR at 4 months showed complete regeneration of the rib in the chest region (Fig. 2f). A CT scan of the head showed that the rib grafts had integrated into the skull and new bony cross-bridge formations were noted 4 months after the procedure (Fig. 8).
Four-month follow-up CT scan showing early bone and cross bridge formation in the axial (a), sagittal (b), and coronal planes (c). Three-dimensional reconstruction of the CT scan showing new bone formation (P posterior, A anterior, L left). Volume rendering was made using Slicer 4.6.2 software). Arrows denote the areas where new bone was noted around the construct (d)
Conclusion
Due to its locally invasive nature, cranial fasciitis is often difficult to differentiate from malignancies and infections. Its nonspecific clinical and radiographic presentation makes complete surgical resection a necessary avenue for diagnosis and cure. Some large lesions can leave a sizeable skull defect behind warranting cranial reconstruction. Allograft reconstruction is superior to titanium mesh or other synthetic materials as cranial fasciitis is a disease of childhood and often presents while the skull continues to grow.
References
Adler R, Wong CA (1986) Cranial fasciitis simulating histiocytosis. J Pediatr 109:85–88
Agozzino M, Cavallero A, Inzani F, Acchiardi I, Locatelli D, Scagnelli P et al (2006) Cranial fasciitis with exclusive intracranial extension in an 8-year-old girl. Acta Neuropathol 111:286–288
Barohn RJ, Kasdon DL (1980) Cranial fasciitis—nodular fasciitis of the head. Surg Neurol 13:283–285
Boddie DE, Distante S, Blaiklock CT (1997) Cranial fasciitis of childhood: an incidental finding of a lytic skull lesion. Br J Neurosurg 11:445–447
Clapp CG, Dodson EE, Pickett BP, Lambert PR (1997) Cranial fasciitis presenting as an external auditory canal mass. Archives of Otolaryngology-Head & Neck Surgery 123:223–225
Coates DB, Faught P, Sadove AM (1990) Cranial fasciitis of childhood. Plast Reconstr Surg 85:602–605
Curtin E, Caird J, Murray DJ (2014) Cranial fasciitis located at the temporal region in a 2-year-old girl. Childs Nerv Syst 30:2163–2167
Delfini R, Broggi G, Chandler WF, McCutcheon IE (2004) Parasagittal cranial fasciitis after irradiation of a cerebellar medulloblastoma: case report—comments. Neurosurgery 54:1266–1267
du Toit LE, Zuhlke AZ, Graewe FR (2009) Cranial fasciitis presenting as a frontonasal mass. Journal of Craniofacial Surgery 20:1197–1199
Eden KC (1939) The benign fibro-osseous tumours of the skull and facial bones. Br J Surg 27:323–350
Edwards MS, Ousterhout DK (1987) Autogeneic skull bone grafts to reconstruct large or complex skull defects in children and adolescents. Neurosurgery 20:273–280
Fissenden TM, Taheri MR, Easley S, Monfared A (2014) Cranial fasciitis of the petrous temporal bone. Int J Pediatr Otorhinolaryngol 78:1430–1432
Foureur N, Enjolras O, Boccon-Gibod L, Wetterwald E, Diner P, Escande JP (2002) Cranial fasciitis of childhood. Annales De Dermatologie Et De Venereologie 129:732–734
Garza L, Allen L, Eghbalieh N, Palacios E, Neitzschman H (2012) Cranial fasciitis of childhood: a lytic skull lesion. J La State Med Soc 164:347–349
Gonzalez-Garcia L, Ros-Lopez B, Weil-Lara B, Perez-da Rosa S, Dominguez-Paez M, Medina-Imbroda JM et al (2013) Cranial fasciitis: a case report and review of the literature. Neurocirugia 24:47–50
Govender PV, Jithoo R, Chrystal V, Dauth T, Nathoo N (2001) Cranial fasciitis—case illustration. J Neurosurg 94:681–681
Halder A, Greene CS, Rivard DC, Shao L (2012) Cranial fasciitis presenting as an intracranial mass in a 10-year-old girl. Pediatr Dev Pathol 15:146–150
Hattab EM, Dvorscak LE, Boaz JC, Douglas AC, Ulbright TM (2014) Parasagittal cranial fasciitis following infratemporal fossa rhabdomyosarcoma. Neuropathology 34:291–294
Hobar PC, Masson JA, Wilson R, Zerwekh J (1996) The importance of the dura in craniofacial surgery. Plast Reconstr Surg 98:217–225
Hoeffel JC (1993) Cranial fasciitis of childhood—a case report. Eur J Pediatr Surg 3:376–376
Hoya K, Usui M, Sugiyama Y, Nagashima K (1996) Cranial fasciitis. Childs Nerv Syst 12:556–558
Hunter NS, Bulas DI, Chadduck WM, Chandra R (1993) Cranial fasciitis of childhood. Pediatr Radiol 23:398–399
Hussein MR (2008) Cranial fasciitis of childhood: a case report and review of literature. J Cutan Pathol 35:212–214
Imafuku S, Takahashi A, Hashizumi Y, Sasamoto K, Tokumaru R, Iwasaki H et al (2011) Cranial fasciitis resembling infantile fibrosarcoma differentiated by genetic assay. J Dermatol 38:1006–1009
Inamura T, Takeshita I, Nishio S, Fujiwara S, Fukui M (1991) Cranial fasciitis—case report. Neurosurgery 28:888–889
Iqbal K, Saqulain G, Udaipurwala IH, Ashraf J, Aijaz F, Jalisi M (1995) Cranial fasciitis—presentation as a postauricular mass. J Laryngol Otol 109:255–257
Johnson KK, Dannenbaum MJ, Bhattacharjee MB, Illner A, Dauser RC, Whitehead WE et al (2008) Diagnosing cranial fasciitis based on distinguishing radiological features. Journal of Neurosurgery-Pediatrics 2:370–374
Keyserling HF, Castillo M, Smith JK (2003) Cranial fasciitis of childhood. Am J Neuroradiol 24:1465–1467
Kumon Y, Sakaki S, Sakoh M, Nakano K, Fukui K, Kurihara K (1992) Cranial fasciitis of childhood—a case report. Surg Neurol 38:68–72
Lang DA, Neil-Dwyer G, Evans BT, Sarsfield P, Nenji E (1996) Cranial fasciitis of the orbit and maxilla: extensive resection and reconstruction. Childs Nerv Syst 12:218–221
Larralde M, Boggio P, Schroh R, Cusumano H (2003) Cranial fasciitis of childhood. Int J Dermatol 42:137–138
Lauer DH, Enzinger FM (1980) Cranial fasciitis of childhood. Cancer 45:401–406
Lecavalier M, Ogilvie LN, Magee F, Poskitt KJ, Kozak FK (2014) Cranial fasciitis: a rare pediatric non-neoplastic lesion with 14-year follow up. Am J Otolaryngol 35:647–650
Lee JY, Kim YC, Shin JH (2004) Cranial fasciitis treated with intrallesional corticosteroids. Int J Dermatol 43:453–455
Lim H, Chung J, Park DH, Yoon SH (2016) Long-term results of remodelling the facial bones with a soft moulding helmet in beagles: the “reciprocally stimulated growth” hypothesis. British Journal of Oral & Maxillofacial Surgery 54:40–45
Longatti P, Marton E, Bonaldi L, Orvieto E (2004) Parasagittal cranial fasciitis after irradiation of a cerebellar medulloblastoma: case report. Neurosurgery 54:1263–1266
Marciano S, Vanel D, Mathieu MC (1999) Cranial fasciitis in an adult: CT and MR imaging findings. Eur Radiol 9:1650–1652
Marshall LR, Salib RJ, Mitchell TE, Moore I (2009) A case of cranial fasciitis masquerading as acute mastoiditis. J Laryngol Otol 123:245–247
Martinez-Lage JF, Torroba A, Lopez F, Monzonis MC, Poza M (1997) Cranial fasciitis of the anterior fontanel. Childs Nerv Syst 13:626–628
Mollejo M, Millan JM, Ballestin C, Serrano C (1990) Cranial fasciitis of childhood with reactive Periostitis. Surg Neurol 33:146–149
Noguchi O, Kuroiwa M, Kogure S, Kohno N, Yoshida K, Zama A et al (1999) Cranial fasciitis of a neonatal case with massive intra- and extracranial extension. Neurol Surg 27:163–169
Oh CK, Whang SM, Kim BG, Ko HC, Lee CH, Kim HJ et al (2007) Congenital cranial fasciitis—“watch and wait” or early intervention. Pediatr Dermatol 24:263–266
Opperman LA, Sweeney TM, Redmon J, Persing JA, Ogle RC (1993) Tissue interactions with underlying dura-mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn 198:312–322
Pagenstecher A, Emmerich B, Vanvelthoven V, Korinthenberg R, Volk B (1995) Exclusively intracranial cranial fasciitis in a child—case report. J Neurosurg 83:744–747
Pasquier B, Keddari E, Pasquier D, Barge M, Bost M, Couderc P (1984) Cranial fasciitis of childhood—a neonatal case with dural involvement. Ann Pathol 4:371–375
Patterson JW, Moran SL, Konerding H (1988) Cranial fasciitis. J Cutan Pathol 15:335–335
Posnick JC, Goldstein JA, Persing JA, Parent AD, Armstrong D, Rutka JT (1993) Reconstruction of skull defects in children and adolescents by the use of fixed cranial bone-grafts—long-term results. Neurosurgery 32:785–791
Rakheja D, Cunningham JC, Mitui M, Patel AS, Tomlinson GE, Weinberg AG (2008) A subset of cranial fasciitis is associated with dysregulation of the Wnt/beta-catenin pathway. Mod Pathol 21:1330–1336
Rapana A, Iaccarino C, Bellotti A, Marsicano C, Donnianni T, Tedeschi E (2002) Exclusively intracranial and cranial fasciitis of the adult age. Clin Neurol Neurosurg 105:35–38
Ringsted J, Ladefoged C (1986) Cranial fasciitis of childhood. Acta Neurol Scand 73:103–103
Sajben FP, Eichenfield LF, O'Grady TC, Cunningham BB (1999) Cranial fasciitis of childhood. Pediatr Dermatol 16:232–234
SantaCruz K, Brace J, Hall W (2007) A case of cranial fasciitis originating within the diploic space of an adult: case report. Neurosurgery 61:E1338; discussion E1338
Sarangarajan R, Dehner LP (1999) Cranial and extracranial fasciitis of childhood: a clinicopathologic and immunohistochemical study. Hum Pathol 30:87–92
Sato Y, Kitamura T, Suganuma Y, Kotani T, Hata J (1993) Cranial fasciitis of childhood—a case report. Eur J Pediatr Surg 3:107–109
Sayama T, Morioka T, Baba T, Ikezaki K, Fukui M (1995) Cranial fasciitis with massive intracranial extension. Childs Nerv Syst 11:242–245
Stal S, Netscher DT, Shenaq S, Spira M (1992) Reconstruction of calvarial defects. South Med J 85:812–819
Summers LE, Florez L, Berberian JM, Bhattacharjee M, Walsh JW (2007) Postoperative cranial fasciitis—report of two cases and review of the literature. J Neurosurg 106:1080–1085
Takeda N, Fujita K, Katayama S, Akutsu N, Hashimoto K, Kohmura E (2008) Cranial fasciitis presenting with intracranial mass: a case report. Pediatr Neurosurg 44:148–152
Wagner RD, Wang EK, Lloyd MS, Lam SK, Khechoyan DY (2016) Cranial fasciitis: a systematic review and diagnostic approach to a pediatric scalp mass. Journal of Craniofacial Surgery 27:E65–E71
Wu B, Zhu H, Liu WD, Chen LY (2013) Occipital diploic cranial fasciitis after radiotherapy for a cerebellar medulloblastoma. Journal of Neurosurgery-Pediatrics 12:637–641
Yebenes M, Gilaberte M, Romani J, Lloreta J, Pujol RM (2007) Cranial fasciitis in an 8-year-old boy: clinical and histopathologic features. Pediatr Dermatol 24:E26–E30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest concerning the materials or methods used in this study or findings specified in the manuscript.
Rights and permissions
About this article
Cite this article
Flouty, O.E., Piscopo, A.J., Holland, M.T. et al. Infantile cranial fasciitis: case-based review and operative technique. Childs Nerv Syst 33, 899–908 (2017). https://doi.org/10.1007/s00381-017-3417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3417-y