Abstract
Introduction
After an endoscopic third ventriculostomy (ETV) fails, it is unclear how well subsequent treatment fares, especially in comparison to shunts inserted as primary treatment. In this study, we present a further analysis of the infants enrolled a prospective multicentre study who failed ETV and describe the outcome of their subsequent treatment, comparing this to those who received shunt as their primary treatment.
Methods
This was a post hoc analysis of data from the International Infant Hydrocephalus Study (IIHS)—a prospective, multicentre study of infants with hydrocephalus from aqueductal stenosis who received either an ETV or shunt. In the current analysis, we compared the results of the 38 infants who failed ETV and the 43 infants who received primary shunt. Patients were followed prospectively for time to treatment failure, defined as the need for repeat CSF diversion procedure (shunt or ETV) or death due to hydrocephalus.
Results
There were a total of 81 patients: 43 primary shunts, 34 shunt post-ETV, and 4 repeat ETV. The median time between the primary ETV and the second intervention was 29 days (IQR 14–69), with no significant difference between repeat ETV and shunt post-ETV. Median length of available follow-up was 800 days (IQR 266–1651), during which time, failure was noted in 3 (75.0%) repeat ETV patients, 10 (29.4%) shunt post-ETV patients, and 9 (20.9%) primary shunt patients. In an adjusted Cox regression model, the risk of failure was higher for repeat ETV compared to primary shunt, but there was no significant difference between primary shunt and shunt post-ETV. No other variable showed statistical significance.
Conclusions
In our prospective study of infants with aqueductal stenosis, there was no significant difference in failure outcome of shunts inserted after a failed ETV and primary shunts. Therefore, our data do not support the notion that previous ETV confers either a protective or negative effect on subsequently-placed shunts. Larger studies, in a wider ranging population, are required to establish how widely these data apply.
Trial registration
NCT00652470
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
We recently reported initial results of the International Infant Hydrocephalus Study (IIHS) [1], an international, prospective, multicentre study that aimed to answer the question: in infants (<24 months old) with symptomatic triventricular hydrocephalus from aqueductal stenosis, does initial treatment with endoscopic third ventriculostomy (ETV) result in superior or no worse outcome at 5 years of age compared to shunt? While the 5 year outcome results are still pending, the initial results demonstrated higher risk of failure after ETV compared to shunt. After failed ETV, however, it is unclear how children do with their subsequent treatment. While there is some data regarding repeat ETV in this context [2–5], there is little regarding the outcome of shunts after a failed ETV [6]. In the current paper, we present a further post hoc analysis of the infants in the IIHS who failed ETV and describe the outcome of their subsequent treatment, comparing it to those who received shunt as their primary treatment.
Methods
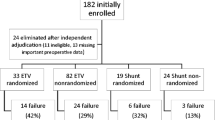
The details of the IIHS have been presented before [1, 7]. Briefly, the IIHS was a prospective study, which contained both a randomized and non-randomized arm [8], and involved centers experienced in pediatric neuroendoscopy. The eligibility criteria were <24 months of age at the time of operation, symptomatic triventricular hydrocephalus (TVH) requiring first treatment, born at >36 weeks gestation, and preoperative MRI showing aqueductal stenosis with no other major brain anomalies. Patients with a history of intraventricular hemorrhage (intra-uterine or post-natal) or intracranial infection were included, unless this related to prematurity. Patients were excluded if they had open spina bifida, Dandy Walker syndrome with vermian agenesis/dysgenesis, perinatal asphyxia, severe brain dysmorphic anatomical features, known chromosomal abnormality, or intracranial tumor. A total of 158 eligible patients were previously analyzed, of whom 115 had ETV as first intervention and 43 had shunt as first intervention. Of the 115 ETV patients, 38 demonstrated treatment failure, as determined by their treating surgeon, and required a second surgical intervention for hydrocephalus. These 38 patients are the focus of the current analysis. At treatment failure, the treating surgeon decided whether to repeat the ETV or insert a shunt. Patients were then followed prospectively.
Subsequent treatment failure was similarly defined as the need for any repeat intervention for definitive CSF diversion (including ETV or shunt insertion/revision), as determined by the treating surgeon, following standard clinical practice, or death related to hydrocephalus.
Analysis
Data are presented as median and inter-quartile range (IQR). Survival curves were constructed using Kaplan-Meier method for time-to-treatment failure and compared using log-rank test. We compared the outcome of “repeat ETV” (i.e., ETV performed again after failed ETV), “shunt post-ETV” (i.e., shunt after failed ETV), and “primary shunt” (i.e., the 43 patients from IIHS who received shunt as their first hydrocephalus treatment). We performed Cox proportional hazards regression to compare time-to-first treatment failure adjusting for patient age (months), history of infection/hemorrhage (yes/no), and geographical continent. Geographical continent was categorized as The Americas (since there were only a few patients from North America alone), Europe, and Asia. Proportional hazards assumption was checked by assessing the significance of each variable as an interaction with time and was confirmed for all variables.
The IIHS was publically registered (NCT00652470) and received ethics approval from all participating institutions. Participating investigators and other trial personnel are listed in the Appendix.
Results
Baseline data for the 38 patients who failed ETV and received subsequent hydrocephalus treatment are shown in Table 1. Of these, 4 underwent repeat ETV and 34 underwent shunt post-ETV. The median time between the primary ETV and the second intervention was 29 days (IQR 14–69), with no significant difference between repeat ETV and shunt post-ETV (Table 1). Table 1 also lists the baseline data for the 43 patients who underwent shunt as their first treatment for hydrocephalus.
Median length of available follow-up was 800 days (IQR 266–1651), during which time, failure was noted in 3 (75.0%) repeat ETV patients, 10 (29.4%) shunt post-ETV patients, and 9 (20.9%) primary shunt patients. Among these failures, there was one hydrocephalus-related death (in the primary shunt group) due to presumed shunt failure in a child who died before being able to be transferred to the treating neurosurgical centre.
Unadjusted survival curves comparing time to first treatment failure for the three groups are shown in Fig. 1. The curves were significantly different (p = 0.02, log-rank). The hazard ratios from the adjusted Cox regression are shown in Table 2. In this adjusted model, the risk of failure was higher for repeat ETV compared to primary shunt, but there was no significant difference between primary shunt and shunt post-ETV. No other variable showed statistical significance.
Discussion
Our prospective multicentre data in infants with TVH show that the failure pattern for shunts inserted at first treatment is similar to those inserted after ETV failure. Although the number of patients with repeat ETV was small, these faired significantly worse.
In the setting of a failed ETV, the decision to proceed with repeat ETV versus shunt is controversial. Several factors can go into this decision-making, including age of the patient, etiology of hydrocephalus, duration between primary ETV and ETV failure, and imaging findings. Some would advocate for repeat ETV, for example, for older patients, those who have had a long duration of success with ETV prior to failure, or in whom MRI shows loss of a previously-visualized flow-void. It has also been postulated that ETV can have a protective effect for a subsequent shunt, resulting a lower failure rate for shunts inserted after a failed ETV. Some have even suggested a synergistic role and have advocated for simultaneous ETV and shunt [9].
There are a number of reports of repeat ETV after an initially failed ETV. In several mixed pediatric and adult series, success for repeat ETV has been reported as between 65 and 100% [2–4]. In perhaps the largest study to date, Marano et al. reported on the experience of repeat ETV in 215 children treated at CURE Children’s Hospital in Uganda [5]. The median age at repeat ETV was 6 months and the estimated 7-year success for repeat ETV was 51%. Longer time to failure of initial ETV, post-infectious etiology and prior choroid plexus cauterization (CPC) had higher chance of success with repeat ETV. In our series, we had only four patients with repeat ETV performed at a median age of 8 months. The success rate was only 25%.
The reported experience with the outcome of shunts after failed ETV is sparser. One of the largest studies also comes from CURE Children’s Hospital in Uganda [6], which compared 255 primary shunts, 370 shunts placed after an abandoned ETV attempt, and 275 shunts placed after a failed ETV (with or without CPC). They found no significant difference in operative mortality or shunt infection among the three groups. Within the post-infectious group only, shunts placed after failed ETV did better, but this was not observed in any other group of patients and was likely a consequence of the timing of shunt placement. Overall, the pattern of shunt failure was similar regardless of previous failed ETV. Our study, although with much smaller numbers, concurs with this result. We observed very similar failure rates for primary shunts and shunt post-ETV (20.9 and 29.4%, respectively), suggesting that there is no protective effect on shunt from a previous ETV.
Our study, however, has some important limitations. First, the sample size is quite small, especially for the repeat ETV group (only four patients). Certainly, for this group, we cannot draw any meaningful conclusions. We also, however, cannot rule out the possibility that our study was under-powered to demonstrate a significant difference in outcome between primary shunts and shunt post-ETV. Second, although we independently adjudicated eligibility criteria for the study, treatment failure was determined solely by the treating surgeon, which can introduce bias. This relates to determining failure of the primary ETV procedure, but also shunt failure. Third, our sample was limited only to infants with aqueductal stenosis. It is unclear if these results are applicable to the broader population of pediatric hydrocephalus. Fourth, virtually all of the 38 ETV failures in our series were relatively early failures, so we cannot comment on the outcome of treatment following delayed ETV failure. Delayed ETV failures are, however, relatively rare occurrences [10, 11].
Conclusions
In our prospective study of infants with aqueductal stenosis, there was no significant difference in failure outcome of shunts inserted after a failed ETV and primary shunts. Therefore, our data do not support the notion that previous ETV confers either a protective or negative effect on subsequently placed shunts. Larger studies, in a wider ranging population, are required to establish how widely these data apply.
References
Kulkarni AV, Sgouros S, Constantini S (2016) International infant hydrocephalus study: initial results of a prospective, multicenter comparison of endoscopic third ventriculostomy (ETV) and shunt for infant hydrocephalus. Childs Nerv Syst 32:1039–1048. doi:10.1007/s00381-016-3095-1
Moreira I, Pereira J, Oliveira J et al (2016) Endoscopic re-opening of third ventriculostomy: case series and review of literature. Clin Neurol Neurosurg 145:58–63. doi:10.1016/j.clineuro.2016.04.007
Siomin V, Weiner H, Wisoff J et al (2001) Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst 17:551–555. doi:10.1007/s003810100475
Surash S, Chumas P, Bhargava D et al (2010) A retrospective analysis of revision endoscopic third ventriculostomy. Childs Nerv Syst 26:1693–1698. doi:10.1007/s00381-010-1176-0
Marano PJ, Stone SSD, Mugamba J et al (2015) Reopening of an obstructed third ventriculostomy: long-term success and factors affecting outcome in 215 infants. J Neurosurg Pediatr 15:399–405. doi:10.3171/2014.10.PEDS14250
Warf BC, Bhai S, Kulkarni AV, Mugamba J (2012) Shunt survival after failed endoscopic treatment of hydrocephalus. J Neurosurg Pediatr 10:463–470. doi:10.3171/2012.9.PEDS1236
Sgouros S, Kulkharni AV, Constantini S (2006) The international infant hydrocephalus study: concept and rational. Childs Nerv Syst 22:338–345. doi:10.1007/s00381-005-1253-y
Olschewski M, Schumacher M, Davis KB (1992) Analysis of randomized and nonrandomized patients in clinical trials using the comprehensive cohort follow-up study design. Control Clin Trials 13:226–239
Shim K-W, Kim D-S, Choi J-U (2008) Simultaneous endoscopic third ventriculostomy and ventriculoperitoneal shunt for infantile hydrocephalus. Childs Nerv Syst 24:443–451. doi:10.1007/s00381-007-0526-z
Drake J, Chumas P, Kestle J et al (2006) Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg 105:118–126. doi:10.3171/ped.2006.105.2.118
Kahle KT, Kulkarni AV, Limbrick DD et al (2016) Hydrocephalus in children. Lancet 387:788–799. doi:10.1016/S0140-6736(15)60694-8
Acknowledgements
The authors would like to extend a special thanks to Adina Sherer, who ran the organizational logistics of this study and without whom the IIHS would not have been possible.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest with respect to this work.
Additional information
See Appendix for full list of study investigators
Appendix: IIHS Personnel
Appendix: IIHS Personnel
Steering Committee: Shlomi Constantini (Principal Investigator), Spyros Sgouros, Abhaya V. Kulkarni
Consultant Neurologist: Yael Leitner
Data Safety Monitoring Committee: John RW Kestle (Chair), Douglas D Cochrane, Maurice Choux, Fleming Gjerris
Coordinating Administrator: Adina Sherer
Participating Investigators (in parentheses are the number of eligible patients contributed to the study by each investigator):
Medical Center | IIHS Participants | # of Patients |
Ankara, Turkey Hacettepe University Hospital | Nejat Akalan, Burçak Bilginer | (12) |
Barcelona, Spain Hospital Sant Joan de Deu | Ramon Navarro (currently at Cleveland Clinic Abu Dhabi, UAE) | (7) |
Belgrade, Serbia | Ljiljana Vujotic | (8) |
Clinical Center of Serbia, Belgrade, Neurosurgery Division | ||
Berlin, Germany Charité - Universitätsmedizin Berlin | Hannes Haberl, Ulrich-Wilhelm Thomale | (4) |
Birmingham, UK Birminghan Children’s Hospital | Spyros Sgouros (currently at "Mitera" Childrens Hospital) | (1) |
Buenos Aires, Argentina | Graciela Zúccaro, Roberto Jaimovitch | (21) |
Hospital De Pediatria Prof. Dr. J.P. Garrahan | ||
Chicago, USA | David Frim, Lori Loftis | (3) |
The University of Chicago Comer Children’s Hospital | ||
Dallas, USA Children's Medical Center of Dallas | Dale M. Swift, Brian Robertson, Lynn Gargan | (6) |
Debrecen, Hungary | László Bognár, László Novák, Georgina Cseke | (5) |
University of Debrecen, Clinical Center, Department of Neurosurgery | ||
Genova, Italy | Armando Cama, Giuseppe Marcello Ravegnani | (3) |
Giannina Gaslini Hospital, Gaslini Children Institute | ||
Giessen/Leipzig University Hospital Gießen and Marburg | Matthias Preuß Currently at University Hospital Leipzig | (4) |
Greifswald, Germany | Henry W. Schroeder, Michael Fritsch, Joerg Baldauf | (2) |
Ernst-Moritz-Arndt-Universität Klinik für Neurochirurgie | ||
Katowice, Poland Medical University of Silesia | Marek Mandera, Jerzy Luszawski, Patrycja Skorupka | (9) |
Liverpool, UK Alder Hey Children’s Hospital | Conor Mallucci, Dawn Williams | (4) |
Lodz, Poland | Krzysztof Zakrzewski, Emilia Nowoslawska | (2) |
Polish Mother’s Memorial Hospital, Research Institute | ||
Lucknow (KGMC), India CSM Medical University (KGMC) | Chhitij Srivastava | (4) |
Lucknow (SGPGI), India | Ashok K. Mahapatra, Raj Kumar, Rabi Narayan Sahu | (8) |
Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGI) | ||
Moscow, Russia Burdenko Neurosurgical Institute | Armen G. Melikian (Армен Меликян), Anton Korshunov (Антон Евгеньевич Коршунов), Anna Galstyan (Анна Галстян) | (11) |
New Delhi, India All India Institute of Medical Sciences | Ashish Suri, Deepak Gupta | (12) |
Nijmegen, The Netherlands Radboud University Medical Center | J. André Grotenhuis, Erik J. van Lindert | (9) |
Nova Lima, Brazil | José Aloysio da Costa Val | (5) |
Neurocirurgia Infantil, Biocor Instituto | ||
Rome, Italy | Concezio Di Rocco, Gianpiero Tamburrini | (4) |
Pediatric Neurosurgery, Policlinico Universitario “A. Gemelli” | ||
São Paulo, Brazil Escola Paulista de Medicina, UNIFESP | Samuel Tau Zymberg, Sergio Cavalheiro | (3) |
Shanghai, China | Ma Jie, Jiang Feng | (3) |
Xinhua Hospital, Shanghai JiaoTong University School of Medicine | ||
Tel Aviv, Israel | Shlomi Constantini, Orna Friedman | (20) |
Dana Children’s Hospital, Tel Aviv Medical Center | ||
Toronto, Canada Hospital for Sick Children | Abhaya V. Kulkarni | (5) |
Warsaw, Poland | Marcin Roszkowski, Slawomir Barszcz | (7) |
Children’s Memorial Health Institute | ||
The following centres (and investigators) participated in the IIHS, but did not enroll any patients: Baltimore, Maryland, USA (George Jallo); Gainesville, Florida, USA (David W. Pincus, Bridget Richter); Kiel, Germany (HM Mehdorn, Susan Schultka); London, Ontario, Canada (Sandrine de Ribaupierre); London, UK (Dominic Thompson, Silvia Gatscher); Mainz, Germany (Wolfgang Wagner, Dorothee Koch); Reggio Calabria, Italy (Saverio Cipri, Claudio Zaccone); Winnipeg, Manitoba, Canada (Patrick McDonald).
Rights and permissions
About this article
Cite this article
Kulkarni, A.V., Sgouros, S., Constantini, S. et al. Outcome of treatment after failed endoscopic third ventriculostomy (ETV) in infants with aqueductal stenosis: results from the International Infant Hydrocephalus Study (IIHS). Childs Nerv Syst 33, 747–752 (2017). https://doi.org/10.1007/s00381-017-3382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3382-5