Abstract
Purpose
Convection-enhanced delivery (CED), a local drug delivery technique, is typically performed as a single session and drug concentrations therefore decline quickly post CED. Prolonged CED (pCED) overcomes this problem by performing a long-term infusion to maintain effective drug concentrations for an extended period. The purpose of the current study was to assess the toxicity of using pCED to deliver single and multi-drug therapy in naïve rat brainstem.
Methods
Sixteen rats underwent pCED of three small-molecule kinase inhibitors in the pons. Single and multi-drug combinations were delivered continuously for 7 days using ALZET mini-osmotic pumps (model 2001, rate of 1 μl/h). Rats were monitored daily for neurological signs of toxicity. Rats were sacrificed 10 days post completion of infusion, and appropriate tissue sections were analyzed for histological signs of toxicity.
Results
Two rats exhibited signs of neurological deficits, which corresponded with diffuse inflammation, necrosis, and parenchymal damage on histological analysis. The remaining rats showed no neurological or histological signs of toxicity.
Conclusion
The neurological deficits in the two rats were likely due to injury from physical force, such as cannula movement post insertion and subsequent encephalitis. The remaining rats showed no toxicity and therefore brainstem targeting using pCED to infuse single and multi-drug therapy was well tolerated in these rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse intrinsic pontine gliomas (DIPGs) are inoperable and lethal high-grade gliomas that account for 75–80 % of pediatric brainstem gliomas [8]. The current standard of care for DIPG is radiation therapy and despite numerous efforts to improve treatment, the prognosis for DIPG remains poor, with median survival of shorter than 1 year [8].
The lack of progress in DIPG therapy can be explained in part by ineffective drug delivery across the intact blood-brain barrier (BBB). Convection-enhanced delivery (CED) is a form of local delivery that utilizes hydraulic pressure to deliver infusate directly to a target region [4]. This method enhances drug distribution and attains high local infusate concentration in the brain [4, 6].
Standard CED is typically performed as a single session and drug concentrations therefore decline quickly post CED, leading to suboptimal therapeutic efficacy. Prolonged CED (pCED) overcomes this problem by performing a long-term infusion to maintain effective drug concentrations for an extended period sufficient for cancer therapy. No study to our knowledge has assessed the toxicity of pCED of chemotherapeutic agents into the rat brainstem.
Recent work has highlighted platelet-derived growth factor receptor (PDGFR), a receptor tyrosine kinase (RTK) that uses phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) as downstream signaling network, as the most commonly overexpressed oncogene in DIPGs [15, 17, 20]. The RTK/PI3K/AKT pathway is responsible for cell growth, proliferation, survival, and angiogenesis and is therefore likely a main driving force of tumorigenesis in DIPG [2, 15, 17, 20], making it a logical target in designing a therapeutic strategy. Accordingly, our study used dasatinib, a RTK inhibitor with strong inhibitory effects on PDGFR, perifosine, an AKT inhibitor, and everolimus, a mammalian target of rapamycin (mTOR) inhibitor, in different combinations. Our study investigated the feasibility, safety, and toxicity of single and multi-drug combinations of small-molecule kinase inhibitors delivered by pCED to the rat brainstem.
Materials and methods
Preparation of small-molecule kinase inhibitors
Dasatinib (Selleck Chemicals, Houston, TX) was dissolved in fresh DMSO as a 10 mM stock solution. Everolimus (Selleck Chemicals) was dissolved in anhydrous ethanol as a 10 mM stock solution. Perifosine (Selleck Chemicals) was dissolved in normal saline as a 10 mM stock solution. All stock solutions were further diluted in artificial cerebrospinal fluid (A-CSF) to working concentrations either in single drug solutions or in combination solutions: dasatinib (2 μM), everolimus (20 μM), and perifosine (0.63 mM). These drug concentrations were 20 times the effective values based on our in vitro studies on mouse brainstem glioma cells (data not shown). All drug solutions were stored at −20 °C until used.
Equipment and pump preparation
ALZET (Cupertino, CA) mini-osmotic pumps (model 2001, rate of 1 μl/h, 7 days) were used with 30-gauge blunt needle cannulas (Plastics One Inc., Roanoke, VA) customized to a needle length of 8.5 mm below pedestal. Pump and cannula were connected using polyvinylchloride (PVC) tubing (Plastics One Inc.). Pumps were filled with infusion solution 24 h prior to use. Pumps and tubing were primed in sterile normal saline solution at 37 °C overnight in order to activate pump before implantation. Each pump had a total volume of 200 μl that was infused over a course of 7 days.
Experimental groups
This study was approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University and was in accordance with the Institute for Laboratory Animal Research guidelines. Sixteen 8–10-week-old naïve female Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing between 188 and 250 g were used for studies. Animals were divided into three experimental groups and one control group, each consisting of four rats (Table 1). Group 1 received dasatinib only, group 2 received dasatinib and everolimus, and group 3 received all three drugs. Control group was infused with freshly prepared artificial cerebrospinal fluid (A-CSF) and 0.01 % Coomassie Blue G-250 dye. Drugs were administered continuously for 7 days. Animals were kept alive for 10 days following the completion of infusion. If any animal showed signs of distress or morbidity during the course of the study, it was sacrificed immediately.
Animal surgery
Animals were anesthetized by inhalational isoflurane and placed on a stereotactic frame (Model 900; David Kopf Instruments, Tujunga, CA). A midline scalp incision (10 mm) was made to expose coronal and sagittal sutures. The animal’s head was leveled within the stereotactic frame using bregma and lambda as reference points. Using a dental drill, a burr hole was made 2.0 mm posterior to lambda and 1.0 mm lateral to sagittal suture. The cannula needle was lowered to a depth of 8.5 mm below the skull to target the pons.
Prior to lowering of cannula, drug-loaded pumps were implanted subcutaneously in the rats’ back and attached with PVC tubing to L-shaped arm of cannula. Following needle insertion, cannula was secured onto the skull using a medical grade UV-light cured adhesive (Star Technology, Waterloo, IN). The scalp was closed and an Elizabethan collar was placed on animals to prevent disturbance of cannula. Animals were allowed to recover from anesthesia and were monitored continuously for the next few hours following surgery.
Neurological assessment
Postoperative monitoring assessed both acute and chronic response to drug treatment as well as tolerance to cannula implantation in the pons. Following the first 24 h, animals were monitored daily for signs or symptoms related to treatment-associated toxicities. All animals surviving the full 10 days after the completion of infusion were considered to have survived chronic therapy.
Animals were weighed every 2 days until sacrifice to monitor weight fluctuations. Daily neurological examinations conducted consisted of basic cranial nerve exam (corneal reflexes and symmetric facial movements), motor and sensory, and general health exams. After 10 days from the completion of infusion, animals were humanely euthanized by intracardiac perfusion under deep anesthesia.
Tissue preparation and histological analysis
The animals underwent intracardiac perfusion fixation with 4 % paraformaldehyde (PFA) under deep anesthesia. After harvest, the brains were further fixed in 10 % zinc formalin at room temperature for 3 days before being processed for paraffin embedding. The appearance of Coomassie Blue dye was used as an indicator of cannula tip in choosing the site of sectioning. Sections were cut at a thickness of 5 μm. Every fifth slide was stained with hematoxylin and eosin (H&E) staining, and select slides were stained using Luxol Fast Blue. Slides were analyzed by a board-certified neuropathologist (EL).
Results
Neurological assessment
There were no intraoperative mortalities and all animals awoke from anesthesia without any immediate neurological symptoms. Initial weight loss occurred in all animals but weight was regained within a few days in most animals (Table 2).
The control group and group 1 showed no signs of clinical toxicity. In group 2, one rat had onset of neurological symptoms 24 h post surgery. This included uneven gaits and left-sided hemiparesis, and progressed to dramatic weight loss, decreased feeding, excretion, and grooming. In group 3, one rat displayed onset of neurological symptoms on day 7 post surgery, with uneven gaits, left-sided hemiparesis, weight loss, decrease in feeding, excretion, and grooming. Both animals were euthanized before the study endpoint.
Histological assessment
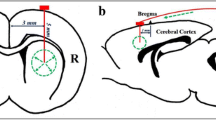
H&E stained slides confirmed that the cannula tip was accurately placed in the rat pons (Fig. 1a). Slides showed inflammation, hemorrhage and gliosis at the site of the cannula tip, and leptomeningeal inflammation in experimental and control animals (Fig. 1b). These histopathological changes were likely due to the surgical procedure. Histological signs of drug toxicity, such as edema, hypoxic damage, cytotoxic damage to neurons, destruction of neurons, demyelination, and neuronal dropout, were not observed in any animal.
a Cannula tip was accurately placed in the pons, and cannula tract with surrounding tissue destruction, chronic lymphocytic infiltration, and b leptomeningeal inflammation (arrow) were observed in all experimental and control animals. These changes were likely due to the surgical procedure and unlikely due to drug toxicity. H&E, original total magnification ×200 (a), ×1000 (b and c)
Two animals, one in group 2 and one in group 3, demonstrated neurological deficits. Histological analysis revealed diffuse necrosis and severe hemorrhage extending into the ventricular system in the rat in group 2 (Fig. 2a, b). Acute inflammation with dense collection of neutrophils was found in the rat in group 3 (Fig. 2c, d). The control group and the remaining experimental animals showed no neurological or histological signs of toxicity.
In group 2 (dasatinib + everolimus), one animal showed neurological deficits which corresponded with a severe hemorrhage (arrow) extending into the ventricular system. Adjacent to the hemorrhage was an area of diffuse necrosis. b Chronic lymphocytic infiltration, visualized in the middle of the image, was also observed. These histological changes and the neurological deficits observed in this animal were likely due to cannula movement post insertion and unlikely due to drug toxicity. In group 3 (dasatinib + everolimus + perifosine), one animal showed neurological deficit which corresponded with a acute inflammation with dense collection of b neutrophils, likely indicating acute encephalitis and unlikely indicating drug toxicity. H&E, original total magnification ×200 (a), ×400 (b), ×400 (c), ×1000 (d)
Discussion
Safety of pCED of small-molecule kinase inhibitors in rat pons
We have demonstrated in the current study that small-molecule kinase inhibitors can be delivered to the rat brainstem via pCED at concentrations high enough to produce glioma cell death in vitro. The majority of animals in our study showed no signs of toxicity at the drug concentrations used. The inflammation, hemorrhage, and gliosis at the site of needle insertion and leptomeningeal inflammation observed in experimental and control animals were likely due to the surgical procedure and unlikely due to drug toxicity.
The likely reason for the observed neurological deficits in the two rats from groups 2 and 3 was due to injury from physical force, such as cannula movement post insertion and subsequent encephalitis, and unlikely due to drug toxicity. Since the control group and the remaining experimental rats showed no neurological or histological signs of toxicity, this demonstrates that brainstem targeting using pCED to infuse single and multi-drug combinations of small-molecule kinase inhibitors was well tolerated in these animals.
Our technique is able to accurately and reproducibly target the rat pons via pCED, as indicated by the Coomassie Brilliant Blue G-250 and histological examination. Coomassie Brilliant Blue G-250 is not toxic at low concentrations, and is rather protective in neurotrauma [16], and might help the needle track heal. Under a trade name, it is also used as a stain to assist ophthalmologists in retinal surgery [12].
Rationale for pCED
Definitive treatment for DIPG is currently lacking and prognosis remains bleak. Therapeutic efficacy in DIPG is limited by the relatively intact BBB, which decreases entry of therapeutic agents into the brain when administered systemically and increases dose-limiting toxicities. Several studies have assessed the interactions between the BBB and chemical compounds, and alternative drug delivery methods [3, 7, 10, 19]. CED is a drug delivery method that circumvents the BBB and delivers therapeutic agents directly at the site of tumor.
One shortcoming of the current application of CED is that it is performed as a single session, and drug concentrations therefore tend to decline quickly post infusion. In published reports of DIPG treatment using CED, investigators reported tumor progression post CED, indicating that single-session CED was not therapeutically efficacious [1, 9]. Furthermore, single-session CED curtails the potential overlap between cancer cell cycling and exposure to therapeutic agents. Lastly, our preliminary studies revealed rapid clearance (<24 h) of agents from the brain interstitium after single-session CED (Zhou et al., unpublished data).
PCED overcomes this issue by performing a long-term local interstitial infusion to maintain effective drug concentrations for an extended period sufficient for cancer therapy. Prolonged drug administration has generated promising results in cancer treatment, especially when combined with molecularly targeted agents [5]. Experimental studies have shown that long-term infusion is promising therapeutically in brain tumor treatment [5, 11, 13, 14, 18]. One group evaluated the safety and feasibility of 10-day intracerebral delivery of topotecan by CED in pigs using a subcutaneously implantable pump. This group concluded that a longer infusion period may lead to better sustained distribution volume and concentration of an agent that is otherwise quickly cleared during shorter infusions [18]. The same group also showed a therapeutic advantage of pCED versus single-session CED in a glioblastoma rat model [11].
This study has demonstrated that pCED of single and multi-drug combinations of small-molecule kinase inhibitors is feasible in the rat brainstem. Future studies will assess the efficacy of pCED using combinatorial-targeted therapy with small-molecule kinase inhibitors in a DIPG mouse model.
References
Anderson RC, Kennedy B, Yanes CL, Garvin J, Needle M, Canoll P, Feldstein NA, Bruce JN (2013) Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr 11:289–295
Becher OJ, Hambardzumyan D, Walker TR, Helmy K, Nazarian J, Albrecht S, Hiner RL, Gall S, Huse JT, Jabado N, MacDonald TJ, Holland EC (2010) Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res 70:2548–2557
Begley DJ (2004) Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther 104:29–45
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91:2076–2080
Boiardi A, Silvani A, Eoli M, Lamperti E, Salmaggi A, Gaviani P, Fiumani A, Botturi A, Falcone C, Solari A, Filippini G, Di Meco F, Broggi G (2008) Treatment of recurrent glioblastoma: can local delivery of mitoxantrone improve survival? J Neuro-Oncol 88:105–113
Groothuis DR, Ward S, Itskovich AC, Dobrescu C, Allen CV, Dills C, Levy RM (1999) Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg 90:321–331
Groothuis DR (2000) The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol 2:45–59
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248
Khatua S, Moore KR, Vats TS, Kestle JR (2011) Diffuse intrinsic pontine glioma-current status and future strategies. Child’s Nerv Syst : ChNS : Off J Int Soc Pediatr Neurosurg 27:1391–1397
Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Fiamengo SA, Neuwelt EA (1998) Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 43:879–886, discussion 886–879
Lopez KA, Tannenbaum AM, Assanah MC, Linskey K, Yun J, Kangarlu A, Gil OD, Canoll P, Bruce JN (2011) Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors. Cancer Res 71:3963–3971
Mennel S, Meyer CH, Schmidt JC, Kaempf S, Thumann G (2008) Trityl dyes patent blue V and brilliant blue G—clinical relevance and in vitro analysis of the function of the outer blood-retinal barrier. Dev Ophthalmol 42:101–114
Park KC, Toyonaga S, Nakabayashi H, Mizobuchi H, Nakai E, Ikawa N, Shimizu K (2004) Primitive neuroectodermal tumor (PNET) treated with interferon-beta after surgical removal and irradiation: case report. Anticancer Res 24:1105–1110
Patchell RA, Regine WF, Ashton P, Tibbs PA, Wilson D, Shappley D, Young B (2002) A phase I trial of continuously infused intratumoral bleomycin for the treatment of recurrent glioblastoma multiforme. J Neuro-Oncol 60:37–42
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS, Onar-Thomas A, Baker JN, Gajjar A, Ellison DW, Baker SJ (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29:3999–4006
Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M (2009) Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A 106:12489–12493
Puget S, Philippe C, Bax DA, Job B, Varlet P, Junier MP, Andreiuolo F, Carvalho D, Reis R, Guerrini-Rousseau L, Roujeau T, Dessen P, Richon C, Lazar V, Le Teuff G, Sainte-Rose C, Geoerger B, Vassal G, Jones C, Grill J (2012) Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One 7:e30313
Sonabend AM, Stuart RM, Yun J, Yanagihara T, Mohajed H, Dashnaw S, Bruce SS, Brown T, Romanov A, Sebastian M, Arias-Mendoza F, Bagiella E, Canoll P, Bruce JN (2011) Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol 13:886–893
Tange Y, Kondo A, Egorin MJ, Mania-Farnell B, Daneriallis GM, Nakazaki H, Sredni ST, Rajaram V, Goldman S, Soares MB, Tomita T (2009) Interstitial continuous infusion therapy in a malignant glioma model in rats. Childs Nerv Syst 25:655–662
Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A, Bouffet E, Hawkins C (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344
Acknowledgments
This study was supported by the New York Community Trust Research Grant and St. Baldrick’s Foundation Summer Fellowship.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ho, S.L., Singh, R., Zhou, Z. et al. Toxicity evaluation of prolonged convection-enhanced delivery of small-molecule kinase inhibitors in naïve rat brainstem. Childs Nerv Syst 31, 221–226 (2015). https://doi.org/10.1007/s00381-014-2568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2568-3