Abstract
Introduction
Medulloblastoma is the most common pediatric central nervous system tumor; however, the causes are not well established. There has been some emphasis on mutations in developmental pathways and their impact on tumor pathology in hereditary diseases, but, in order to better understand the nature of diseases like medulloblastoma, other mechanisms also require attention.
Purpose
The purpose of this review is to provide an overview of the main genes involved in neurodevelopment, their downstream targets, and modulatory links by growth factors. Occurrence of pediatric brain tumors including medulloblastoma are mostly sporadic, but some hereditary diseases like Li–Fraumeni syndrome, Gorlin’s syndrome, Turcot’s syndrome, and Rubenstein–Tarbi syndrome are known to contribute their development as consequences of germline mutations at specific points: DNA-repairing gene Tp53 for Li–Fraumeni syndrome or Patch for Gorlin’s, and apoptosis-related gene product adenomatous polyposis coli for Turcot’s disease.
Conclusion
Intracellular relations at molecular level and future therapeutics that specifically target the corresponding pathways should be well understood in order to prevent and cure childhood medulloblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is the most frequent primary central nervous system (CNS) tumor which is observed in 20–30 % of children up to 10 years old and occurs in the posterior fossa and the fourth ventricle [24,41,61]. The most common location of the tumor is the cerebellum (94.4 %), usually in the midline (75 %) of the inferior vermis [18]. It is neuroectodermal in histology and origin, therefore considered as a member of primitive neuroectodermal embryogenic tumor (PNETs). Approximately 20 % of the children diagnosed with CNS malignancies have MB. Occurrence of pediatric brain tumors including medulloblastoma are mostly sporadic, but some hereditary diseases like Li–Fraumeni syndrome, Gorlin’s syndrome, Turcot’s syndrome, and Rubenstein–Tarbi syndrome are known to contribute their development as consequences of germline mutations at, for example, Tp53 (for Li–Fraumeni), adenomatous polyposis coli (APC, for Turcot), or Ptch (for Gorlin’s). The predispositions underlying MB development are related with developmental signaling pathways like Shh, Wnt/β-catenin, and Notch which are also affiliated to familial inheritance. In addition, aberrant regulation of insulin-like growth factor (IGF) and DNA repair signaling have been implicated in MB formation [11,24,40,42,45,48,65].
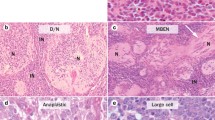
Definitive diagnosis of MB is dependent on tumor histology, and WHO classifies MB according to the histopathology of the tumor: subgroups are defined as classical, desmoplastic/nodular, extensive nodular, anaplastic, and large cell MB. A variant of MB named medullomyoblastoma may exist showing characteristics of rhabdomyoblastic differentiation. MBs display hypercellularity, variable enhancement, and necrotic areas in imaging studies [12,19,26,42,61].
Similarity of cancer stem cells to developing cerebellum cells
Adult neural stem cells are responsible for self-renewal and differentiation into all kinds of neural cells including neurons, astrocytes, and oligodendrocytes. They are found in the subventricular zone of lateral ventricle and subgranular zone of the dentate gyrus [43]. Stem cells are described as self-renewing multipotent cells which display multilineage differentiation. Normal somatic stem cells should renew, and there should be a balance between self-renewal and differentiation in a context similar to cancer. Cancer may be defined as a disorder of unregulated self-renewal. MB arises as a result of dysregulations in differentiation. During these processes, neural stem cells and their progenitors seem to be responsible for transformation in the architecture of brain tumors. Microarray studies suggested a similarity of human MBs with the developing brain. MBs arise from the external granular layer (EGL) of a developing cerebellum. Since some types of human MB exhibit similar gene expression representations to murine EGL, a correlation between stem and progenitor cells, with disrupted or mutated signaling pathways which normally check self-renewal of stem cell such as Shh pathway, as a model of MB development is proposed [19,39,52].
The stem cell theory for carcinogenesis states that cancer cells are produced monoclonally from a stem cell or stem-like cell. Different than normal stem cells, they are supposed to undergo cell division without differentiating terminally [58]. Cancer stem cells were observed in the subgroup of cancer cells which expressed Cluster of Differentiation (CD) 133. When CD 133 (+) human glioma cells were transplanted into the brains of immunodeficient mice, glioma development started. On the other hand, transplantation of CD 133 (−) did not cause tumor formation. This is considered as a sign of self-renewal property [33,39]. But all the tumorigenic cells do not have to be CD 133 (+); they are rather thought to include a different subgroup of stem-like cells [37]. It is also a possibility that some MBs may arise from fully differentiated cells [19].
Roles of developmental pathways in MB formation
Hedgehog-Patch pathways
Oncogenic mutations of elements of Hedgehog-Patch pathway (Hh) has been defined in 20–25 % of human MBs. Hh pathway members are evolutionarily conserved among species varying from Drosophila to humans. The role of Hh pathway, in general, is patterning and developing different organs and, in particular, regulating proliferation of granule neuron precursors (CGNPs). An imbalance between differentiation and proliferation of GNPs is enough to be considered as a cause of MB formation. The cerebellum of mammalians evolves from GNPs at the fetal rhombic lip. Those precursors migrate from the rhombic lip to create the EGL. The relevant Hh subtype is Shh. Interestingly, when downstream targets of Hh pathway are analyzed, it is observed that most of the tumors, which compromise mutated Hh signaling pathway genes, developed additional ways for that pathway activation [23,31,40,48,57].
Hedgehog-patched pathways control cerebellar EGL development. Shh is produced by Purkinje cells and is a target for Patched 1 (Ptch 1). Ptch 1 is a tumor-suppressor gene which codes for a 12-cross occupying transmembrane surface receptor responsible for hedgehog proteins. The location of the chromosome mutated in Gorlin’s syndrome is 9q22-31, which is a potential tumor-suppressor locus. MBs have been proved to evolve from mutated Ptch receptor and protein at about 15 % of sporadic human and mice MBs leading to activation of Hh pathway. Mutational analysis through this locus revealed exon 13 Y601X nonsense mutations in a 3-year-old patient. In the same context, Ptch 1 knockout transgenic mice models have been shown as useful tools to explore MB development [9,15,19,48].
Moreover, Hh signaling cascade is a regulator of DNA-damage response, which is supposed to be important in creating a tendency to medulloblastomagenesis in response to injuries of the genetic material, especially to double-strand breaks (DSBs) when it fails to work properly. The appropriate function of DSBs is to stimulate signaling on purpose to end the damage or the survival of cell [48].
Regulation of Patch expression and how this regulation can contribute to MB formation
The mechanism responsible for related proliferation of GCPs is the inhibition of Smoothened (Smo) function and activation of Gli family of transcription factors including Gli 1, Gli 2, and Gli 3 [11,26]. Gli proteins interact with a cis-element with the aid of a zinc-finger DNA-binding domain, resulting in the activation or inhibition of target genes among which Ptch 1 and Gli transcription factors themselves exist and, therefore, alter features of cells. Gli 1 is always an activator, and its degradation is ubiquitin-mediated [13,36,48,63]. Gli 1 and Gli 2 are found to be more related with tumor development. Numb and Itch proteins mediate the activity of Gli 1. Itch itself is an ubiquitin ligase. Hh/Gli oncogenic potential is affected by the stability of Gli and its interaction with those mediators, Numb and Itch. Dysregulation of Gli 1 by these factors determines the enhancement of Gli 1-dependent proliferation of MB. Negative regulators of the Hedgehog-Patched pathway include Suppressor of the Fused (Sufu), Rab 23, or Ren. Ren deletion at about 40 % of sporadic human MB has been shown to exist. Within sporadic MBs, Ptch 1 loss-of-function mutations are found at a range of 10–20 %. Smo and Sufu mutations are observed in human MB though rare [13,26,48,57]. Smo is a heterotrimeric guanosine triphosphate-binding protein (G-protein)-coupled receptor and is a component of Hh pathway [6,48]. Degradation of Sufu is promoted by Shh via an ubiquitin–proteasome-dependent manner. It has been suggested to underlie to the decreased stability of Sufu in tumor cell lines [63].
Another transcription factor, Nanog, lies in the basis of self-renewal characteristics of embryonic stem cells and has been stressed in the literature by its activation reinitiated by cancer. Po and colleagues showed that the increased expression of Nanog and Gli 1 in either mice or human MB stem cells may be Hh-dependent [46].
ErbB receptor and Patch
The Erb B receptor family has roles implicated for both tumorigenesis and developmental processes. The Erb B4 is highly expressed in human and mice cerebellum during embryogenesis. Its expression is lower during the lifetime of an adult. It has been shown that the expression of cytoplasmic isoforms of ErbB4, CYT 1, and CYT 2, localized in mouse GCPs in vitro and human MB cell lines, falls down by Hh signaling. Selective overexpression of ErbB4 CYT 1 has been observed during the Ptch 1 single mutant studies, which led researchers to propose that there is an alternative tumorigenic pathway valid in the absence of Hh. This overexpression is a protective mechanism to escape from apoptosis [21]. A family of epidermal growth factor receptor ErbB 2, also named HER 2/neu, has been found to be overexpressed at about 80 % of MB specimens representing a bad prognosis [26].
Other regulators of Hh pathway in MB
A transcription factor named Atoh 1 or Math 1 is one of the regulators of Shh pathway having roles in GNP expansion. That transcription factor has been found to be associated with the aggressiveness of MB and bad prognosis. Flora and colleagues suggested that it is important in postpartum cerebellar physiology, which is the factor contributing to MB generation, and actor through activation of Gli 2 [23].
Wnt pathway
Wnt pathway controls stem and progenitor cell proliferations in the early ventral zone (VZ) of the embryo and postpartum SVZ and hippocampus. APC is a gene involved in Wnt pathway which is mutated in Turcot’s syndrome. APC is involved in a complex of proteins consisting β-catenin, glycogen synthase kinase 3-β (GSK3-β), and Axin. Through a recent experiment, it has been revealed that GSK3-β is the pivot molecule interconnecting PI3K/Akt and Wnt pathways for extension of MB [62]; but targets of Wnt pathway are rather considered to be c-Myc, cyclin D1, and AXIN 2. Among them, cyclin D1 is essential in mediating CGCP proliferation and is a downstream target of Shh, Notch, and Wnt signaling. Gain of function mutations in Wnt pathway may also occur. Those mutations seem most likely to be in β-catenin gene but may also be observed in APC and AXIN 2 genes. Axin 2 is defined as a negative loop in Wnt signaling. The APC-Axin complex is primarily responsible for targeting β-catenin to proteolysis by proteasome pathway. Wnt is not considered to control the way that EGL precursors behave [11,26].
Notch pathway
Notch is a transmembrane receptor which is highly conserved. It acts during pattern formation, proliferation, and survival of stem cells but at the meantime inhibits their differentiation. When Notch signaling is impaired, the whole cell field may turn to neurons. Notch signaling results in transcriptional activation of Hes 1 and Hes 5, the helix-loop-helix transcription factors. Notch 2, which is predominantly expressed in proliferating CGNPs, is found to be overexpressed in MBs of about 15 % [11,12,17,26].
It has been demonstrated that inhibiting both Notch and Shh pathways in human primary and cultured cells and the xenografts resulted in more tumor loss than targeting only one of them. It has been declared that Notch signaling is essential for MB survival [28]. However, it has later been reported that Notch signaling is not essential for the pathology of Shh-driven, genetically engineered MB mouse models [30].
Receptor tyrosine kinases and intracellular signaling mechanisms related to MB development
Receptor tyrosine kinases (RTKs) have intrinsic tyrosine kinase activity. They are involved in a variety of cellular processes like cell proliferation and migration, metabolism, and survival, and are activated by either growth promoting factors or cytokines. IGF and cognate receptors (IGF-R) have been found to be responsible for malignancy of MB [26]. The IGF/PI3K signaling pathway enhances the Shh-related MB development. PI3K prevents the degradation of n-Myc; therefore, n-Myc is upregulated. PI3K inhibitors are used to prevent tumor resistance as therapeutic strategies. The function of Myc family of proto-oncogenes is to serve for the regulation of cellular proliferation, differentiation, or apoptosis. An augmentation in Myc transcription has been found to be correlated with decreased survival. Also, inhibition of IGF-1R signaling exhibited reduced MB growth. IGF-1R signaling is thought to activate AKT and PI3K and also Ras/mitogen-activated protein kinase (MAPK) signaling. Within MBs, targets of Ras/MAPK pathways are upregulated. Among the targets of this pathway lies platelet-derived growth factor receptor-B (PDGFR-B) which is upregulated in metastatic MB [3,6,8,11,65].
It has been mentioned that PDGFR is overexpressed especially during metastatic conditions. They treated metastatic MB cells with PDGF and observed that extracellular signal-regulated kinase (ERK) phosphorylation is triggered and migration is advanced. ERK is a member of MAPK family that has been stated to be involved in many steps of tumor progression including regulations of apoptosis-related proteins and extracellular matrix proteins [35,62].
ERK signaling
Downstream targets of MAPK may include mammalian target of the rapamycine (mTOR). It is able to phosphorylate and activate Akt at serine 473. Protein translation that has been regulated by mTOR may lead to malignant growth in case of MBs [3,60].
Rac 1 is a previously defined GTPase belonging to the Rho family as participating in activation of three MAP kinase cascades: ERK, JNK, and p38. Inhibiting them, as a part of the group’s study, revealed ERK and JNK, which are important for invasion and prohibition of growth despite the fact that the prohibitory effect of Rac 1 in one of these cell lines was not found to be intervened by either ERK or JNK. Three different human MB cell lines were tested to evaluate the role of Rac 1 and related signaling molecules as candidates responsible for tumor invasion. They concluded that Rac 1 depletion prevents invasive action but has no effect on cell survival. [64].
Akt signaling
The cascade of PI3K/Akt can be downregulated by PTEN whose homozygous deletions in mice are shown to exhibit dysplasia of primary granule cells at cerebellum [4].
Other RTKs
Impaired regulations of several other RTKs such as c-Met, ErbB 2 (also called Her 2/neu, a family of epidermal growth factor receptor), and vascular endothelial growth factor receptor have also been found to be in association with MB [28]. ErbB 2 has been found to be overexpressed at about 80 % of MB specimens representing a bad prognosis [12,50].
The proto-oncogene kit is a RTK subclass III family member. The amplification of kit is observed in correlation with poor prognosis at some medulloblastoma and cellular PNET species. However, methylation of kit promoter is searched experimentally to support the hypothesis which declares its contribution to tumor formation in MB. This hypothesis has not been proved [16].
Gap junctions in MB development
Gap junctions are channels spanning the cell membrane and are responsible for communication of neighbor cells via permission of direct transport of molecules smaller than 1 kDa. Human cancers are considered to have accumulation of gap junction disruptions, either by a decrease in the expression of channel protein connexin or by a dysfunction of channel creation. It has also been observed that healing the functions of gap junctions reveal a decline in carcinogenic potential [32]. Direct relation of any disruption with functions of gap junctions specifically contributing to MB development requires more experimental evidence.
Neurotrophins in MB development
NTs are members of neurotrophic factors that play important roles in proliferation, migration, and differentiation concerning both developing nervous system and functioning adult nervous system. Maintenance of neuronal activity and regeneration, structural stability, and neurogenesis all depend on normal actions of NTs. Their insufficiency or pathology might result in a disruption of neuronal maintenance, abnormalities within the structure of the brain, and a decrease in plasticity [14,38,51]. In particular, they serve as important modulators of synaptic transmission comprising neurotransmitter release and postsynaptic responses to neurotransmitters, facilitation of long-term potentiation, and survival. Four main NTs in mammals, named nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT 3), and NT 4/5, bind respectively to four receptors named pan 75 neurotrophin receptor (p75NTR), TrkA, TrkB, and TrkC. Trk symbolizes tropomyosin-related kinase family of RTKs. NGF specifically binds to TrkA, BDNF and NT 4 bind to TrkB, NT 3 binds to TrkC but is also able to bind to Trk A and B though with low affinity. When a neurotrophin binds to its receptor, Trk receptor dimerizes, and its intracellular tyrosine residues are phosphorylated. Intracellular signaling pathways are activated thereafter. On the other hand, entire NTs are capable of binding p75NTR [14,49,53,54].
NGF is a neurotrophin which is supposed to be a factor affecting the physical characteristics of MB. Concluding effects of NGF binding to receptor TrkA, like differentiation or proliferation, may involve different cascades depending on the type of cells. In MB cell cultures, NGF has been shown to induce apoptosis which is dependent to Ras activity but independent to MEK/ERK [38]. Also, affluence of TrkC mRNA expression has been correlated with an improved response to the treatment of childhood MB and 5-year survival; however, a study in 2004 has implicated that it had no effect on prognosis [7,12,26].
Similarly, BDNF/TrkB signaling components have been tested for vitality in human MB cells. They also have been noted as possible mediators of durability of cells. Expressions of many microribonucleic acids have been found to be decreased in human MB primary specimens. Especially miR-9 and miR-125 seemed to contribute to growth blockage and promotion of apoptosis after recovery of their expression when the target is a preproliferative TrkC isoform [22,27,50].
Other molecules and pathways related to MB development
c-Myc
Cellular-Myc (c-Myc) is a transcription factor organizing cellular growth and death. It is specifically important for MB because it stands for large-cell/anaplastic subtype and poor prognosis. It is also usually overexpressed or amplified. It has been suggested that pathogenesis of neuronal Myc (n-Myc) involves cooperation of both Shh and c-Myc [11,17,26,65]. Myc is described as an interconnecting agent which settles disarrayed activities of Shh, Wnt, and PI3K/Akt signalings. The transcription factor RE-1 silencing transcription factor is a blocker of neuronal differentiation and is greatly expressed in MB as well as neural stem cells. It is able to transform c-myc-immortalized GCPs towards MB [3,19].
Hippo
Hippo, which is a novel tumor suppressor, limits the organ size by blocking yes-associated protein (YAP 1) transcriptional coactivator activity. YAP 1 is able to cause transformation and proliferation. It acts as a regulator of expression of many transcription factors including RUNX 2, SMAD 7, p73, p53BP2, and TEA domain-associated transcription factor (TEAD) family members. It has been found that YAP 1 and its partner TEAD 1 are expressed in very large amounts in Shh-driven MBs in humans and mice. YAP 1 activity, in cooperation with TEAD 1, leads to proliferation of CGNPs. The YAP 1/TEAD 1 complex adjusts expression of Gli 2, which further translocates to the nucleus and arranges transcription of Gli 1 and then regulates activity of elements of cell cycle machinery [20].
DNA repair mechanism
DNA damage repair or failure, such as deletion of DNA ligase IV, or cell cycle dysregulation influencing components like Kip 1, Ink 4C, Ink 4D, or even loss of genes related with poly(ADP-ribose) polymerase 1, XRCCA, Brca 2Nestin-cre (inactivation by Nestin-cre), have been shown to develop murine MB when combined with p53 deletions. But, in humans, concurrent mutations within these pathways are not frequent [25,28,48]. When Ptc1 heterozygous mice are exposed to a radiation-like environmental factor combined with loss of PARP 1, which is a part of DNA repair mechanism, an increased tendency to develop MB was observed [56].
Hox genes
Homeobox (Hox) complex have differential roles in regulating developmental anteroposterior axis formation which also compromises neuronal classification. This process requires expression of particular genes that belong to and represent the complex [1]. An overexpression and occasionally an amplification of a transcription factor named orthodenticle homeobox 2 (Otx 2) has previously been underlined for MBs. Investigations of the downstream components of Otx 2 actions led people to mention about some cell cycle genes which demonstrate a requirement for participation of other oncogenic changes for MB formation [5].
Other genes and transcription factors
Within classical MBs, SOX gene family members, primarily Sox4 and Sox11, are shown to be overexpressed when compared with ependymoma and normal cerebellum [10,11]. Signal transducer and activator of transcription (STAT) is a transcription factor emphasized as both a downstream of IGF and an inducer of stem-like behavior in glioblastoma-like brain tumors [37].
Role of primary cilia
The possibility of existence of primary cilia in few human MB subtypes has been reported in the literature. Interestingly, those having primary cilia were exhibiting high Hh signaling with defined mutations in pathway components like Ptch1. Experimental mutations using mouse models were performed, and it was concluded that primary cilia are able to activate or inhibit development of the tumors consistent with predisposing oncogenic circumstances [29].
Viral etiology
Associations with viral infections, especially with human neurotrophic polyoma virus (JCV) in MB development have also been described. The viral early protein, T antigen, is thought to be responsible for tumorigenesis. It is observed that transgenic mice producing T antigen in relation with JCV early promoters/enhancers developed neuroblastomas and MBs [47].
Novel chemotherapeutic agents as candidates to prevent MB development
Classical chemotherapeutic agents which are used in the treatment of childhood MB are reviewed before [8]. However, classical chemotherapy is toxic. Toxicity increases when classical drugs are combined with standard surgery and radiotherapy [55]. Basic candidate molecules for a better management of MB in the literature are updated below.
Hedgehog inhibitors
Hedgehog inhibitors are on trial, but there is lack of evidence about their potential clinical use. Most of them target Smo and contribute to silencing of Hh signaling. Antagonizing Smo receptor is shown to inhibit Hh signaling and promote tumor regression within animal models of MB [6,8,26]. According to the cancer stem-cell approach, the cells inside a neoplasm are not equal in unlimited self-renewal potentials and long-term growth. In case of MB, a clinical phenotype is suggested to be affected from modulation of stem cell-like cells. Hh inhibition, for example via cyclopamine, seems to be a potential in vitro MB growth attenuator, but the involvement of stem-like cells in the tumor through this attenuation has not been proved [19]. Existence of studies showing that Shh cascade and errors derived from it does not make significant differences in the prognosis; a resistance to Hh-inhibiting therapies in the literature may require further attention, and understanding on the etiology of MB also regarding the interactions of developmental pathways to propose key downstream targets is needed [44,45].
Wnt inhibitors
Interestingly, phenotypes of blastoma with positive mutations in β-catenin, a member of APC complex functioning at downstream Wnt, are shown to be related with overall survivals. Due to this fact and the suspicions regarding the importance of Wnt in cancer stem cells, it was previously proposed that targeting inhibition of Wnt pathway members may not be beneficial [26].
Notch inhibitors
In MB stem cell lines and PNETs, blocking Notch resulted in suppression of transcription of its target Hes 1 and therefore resulted in exiting from cell cycle, apoptosis, and differentiation. This finding correlated with poor consequences [19,26].
Other inhibitors related with cell signaling
Cascades regulated by Rac 1 have been suggested as potential targets for treatment of MB, but further experimentation is necessary [64]. An ErbB 2-antibody, Trastuzumab, had promising effects to ErbB 2-receptor-positive MB cases, but its activity was limited due to low blood–brain barrier infiltration [26].
2-Amino-N-(4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl)-acetamide (OSU03012) is the third generation cognate of celecoxib and has inhibitory impacts on phosphoinositide-dependent protein kinase 1, the downstream component of PI3K/Akt cascade. OSU03012 triggered apoptosis following interruption of cell division. It has been tested by various ways including combinations with standard chemotherapy agents and the mTOR inhibitors, CCI-779 or rapamycin. The results portrayed its aid in conveying cellular toxicity [4].
Curcumin
An Indian spice product, curcumin, is a polyphenol which has antitumorigenic effects. It is still on the phase of human clinical trials, but its way of action is well proposed including IGF, Akt, MAPK, STAT 3, nuclear factor kappa B, and Notch as target molecules whose dysregulations contribute pathologies of malign brain tumors. Administration of curcumin nanoparticles to MB cell lines was revealed with 35 % inhibition of tumor growth which is further experimented to define potential responsible mechanisms and led to identify increases in apoptosis and cell cycle arrest. Although some MB cell lines display STAT 3α-positive expression pattern, effects of nanocurcumin on STAT signaling has different conclusions depending on the type of lines, but other pathways like IGF are shown to be suppressed by it [37].
PGE2
Prostaglandin E2 (PGE2) was shown to trigger MB growth; therefore, nonsteroid anti-inflammatory drugs and small interfering RNA aiming to inhibit cyclooxygenase 2, which is the producer of PGE2, were administered to human MB cell lines, and a reduction in MB tumor growth was observed. A combinatory treatment approach involving both Shh-like developmental inhibition and application of nonsteroid anti-inflammatory drugs has recently been proposed [2,3].
Superoxide dismutase mimetics
A compound mimicking action of superoxide dismutase in mitochondria called ortho-isomer manganese (III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin has recently been tested in brain tumors including MB. The results point this compound as a strong alternative chemotherapeutic candidate for increasing general survival ratio of 173 % in pediatric MB xenografted mice. The proposed ways of functions include inhibitions of cellular radicals which then affect transcriptional activities and coupling of reductants with relevant signaling molecules [34].
Retinoic acid
Experiments revealed prevention of MB growth by all Trans and 13-cis retinoic acids in vitro, proposing those candidates as drugs to be developed. Retinoids trigger apoptosis and differentiation in mice and cell cultures. Combination trials with other chemotherapeutics are on discussion [8,55,59].
Conclusion
As known today, there are multiple pathways which are responsible for MB formation, most of which are developmental and make crosstalk at certain molecules. Causes of MB still need further investments and investigations. The better nature of the disease is understood, the more effective and lifesaving treatment strategies can be developed. Targeting, for example, elements of MAPK cascades does not seem to promote survival more than classical chemotherapeutics; they both have serious side effects. In case of Shh pathway, it looks a better approach to target and specifically inhibit it, since it may be less active in normal life than development; but at this point, patients are mostly children, who still develop. Again, side effects may prevent a complete cure. Also, tumors may activate alternative pathways to favor their proliferation as usually seen in escaping from the immune system and developing resistance to cytotoxic drugs. As described in the literature above, once the MBs develop, therapy should be personalized, relying on the nature of the tumor with appropriate markers screened. Therefore, the best approach could be the preventive one: by encouraging women to take as many precautions as possible during their pregnancy to at least decrease the frequency of MB.
References
Alberts B, Johnson A, Lewis J et al (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Baryawno N, Sveinbjörnsson B, Eksborg S, Orrego A, Segerström L, Oqvist CO et al (2008) Tumor growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro Oncol 10(5):661–674
Baryawno N, Sveinbjörnson KP, Johnsen JI (2010) Medulloblastoma—a disease with disorganized developmental signaling cascades. Cell Cycle 9(13):2548–2554
Baryawno N, Sveinbjörnsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI (2010) Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res 70(1):266–276
Bunt J, de Haas TG, Hasselt NE, Zwijnenburg DA, Koster J, Versteeg R, Kool M (2010) Regulation of cell cycle genes and induction of senescence by overexpression of OTX2 in medulloblastoma cell lines. Mol Cancer Res 8(10):1344–1357
Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A et al (2010) Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med 29;2(51):51ra70
Chou TT, Trojanowski JQ, Lee VM (2000) A novel apoptotic pathway induced by nerve growth factor-mediated TrkA activation in medulloblastoma. J Biol Chem 275(1):565–570
Crawford JR, MacDonald TJ, Packer RJ (2007) Medulloblastoma in childhood: new biological advances. Lancet Neurol 6(12):1073–1085
Crawford JR, Rood BR, Rossi CT, Vezina G (2009) Medulloblastoma associated with novel PTCH mutation as primary manifestation of Gorlin syndrome. Neurology 72(18):1618
de Bont JM, Kros JM, Passier MM, Reddingius RE, Sillevis Smitt PA, Luider TM et al (2008) Differential expression and prognostic significance of SOX genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro Oncol 10(5):648–660
de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R (2008) Biological background of pediatric medulloblastoma and ependymoma: a review from a translational research perspective. Neuro Oncol 10(6):1040–1060
Dhall G (2009) Medulloblastoma. J Child Neurol 24(11):1418–1430
Di Marcotullio L, Greco A, Mazzà D, Canettieri G, Pietrosanti L, Infante P et al (2011) Numb activates the E3 ligase Itch to control Gli1 function through a novel degradation signal. Oncogene 30(1):65–76
Dwivedi Y (2009) Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat 5:433–449
Ecke I, Petry F, Rosenberger A, Tauber S, Mönkemeyer S, Hess I (2009) Antitumor effects of a combined 5-aza-2'deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res 1;69(3):887–895
Enguita-Germán M, Gurrea M, Schiapparelli P, Zhu TS, Crowley JG, Hamm LL et al (2010) KIT expression and methylation in medulloblastoma and PNET cell lines and tumors. J Neurooncol 103(2):247–253
Epstein RJ (2003) Human molecular biology: An introduction to the molecular basis of health and disease. Cambridge University Press, UK
Eran A, Ozturk A, Aygun N, Izbudak I (2010) Medulloblastoma: a typical CT and MRI findings in children. Pediatr Radiol 40(7):1254–1262
Fan X, Eberhart CG (2008) Medulloblastoma stem cells. J Clin Oncol 26(17):2821–2827
Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S et al (2009) YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev 1; 23(23):2729–2741
Ferretti E, Di Marcotullio L, Gessi M, Mattei T, Greco A, Po A et al (2006) Alternative splicing of the ErbB-4 cytoplasmic domain and its regulation by hedgehog signaling identify distinct medulloblastoma subsets. Oncogene 25:7267–7273
Ferretti E, De Smaele E, Po A, Di Marcotullio L, Tosi E, Espinola MS et al (2009) MicroRNA profiling in human medulloblastoma. Int J Cancer 124(3):568–577
Flora A, Klisch TJ, Schuster G, Zoghbi HY (2009) Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science 326(5972):1424–1427
Fossati P, Ricardi U, Orecchia R (2009) Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev 35:79–96
Frappart PO, Lee Y, Russell HR, Chalhoub N, Wang YD, Orii KE et al (2009) Recurrent genomic alterations characterize medulloblastoma arising from DNA double-strand break repair deficiency. Proc Natl Acad Sci U S A 106(6):1880–1885
Guessous F, Li Y, Abounader R (2008) Signaling pathways in medulloblastoma. J Cell Physiol 217:577–583
Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D et al (2010) microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle 9(6):1031–1036
Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA et al (2004) The SmoA1 mouse model reveals that Notch signaling is critical for the growth and survival of Sonic-Hedgehog induced medulloblastomas. Cancer Res 64:7794–7800
Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez Buylla A (2009) Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 15(9):1062–1065
Hatton BA, Villavicencio EH, Pritchard J, LeBlanc M, Hansen S, Ulrich M et al (2010) Notch signaling is not essential in sonic hedgehog-activated medulloblastoma. Oncogene 29(26):3865–3872
Heretsch P, Tzagkaroulaki L, Giannis A (2010) Modulators of the hedgehog signaling pathway. Bioorg Med Chem 18:6613–6624
Ito S, Hyodo T, Hasegawa H, Yuan H, Hamaguchi M, Senga T (2010) PI3K/Akt signaling is involved in the disruption of gap junctional communication caused by v-Src and TNF-α. Biochem Biophys Res 400(2):230–235
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355(12):1253–1261
Keir ST, Dewhirst MW, Kirkpatrick JP, Bigner DD, Batinic-Haberle I (2011) Cellular redox modulator, ortho Mn(III) meso-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin, MnTnHex-2-PyP(5+) in the treatment of brain tumors. Anticancer Agents Med Chem 11(2):202–212
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802(4):396–405
Lee EY, Ji H, Ouyang Z, Zhou B, Ma W, Vokes SA et al (2010) Hedgehog pathway-regulated gene networks in cerebellum development and tumorigenesis. Proc Natl Acad Sci U S A 107(21):9736–9741
Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG (2011) A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther 11(5):464–473
Lindsay RM (1996) Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: an overview. Philos Trans R Soc Lond B Biol Sci 351(1338):365–373
Lobo NA, Shimono Y, Qian D, Clarke MF (2007) The biology of cancer stem cells. Annu Rev Cell Dev Biol 23:675–699
Michael LE, Westerman BA, Ermilov AN, Wang A, Ferris J, Liu J et al (2008) Bmi1 is required for hedgehog pathway-driven medullablastoma expansion. Neoplasia 10(12):1343–1349
Packer RJ, Cogen P, Vezina G, Rorke LB (1999) Medulloblastoma: clinical and biologic aspects. Neuro Oncol 1(3):232–250
Papaioannou G, Sebire NJ, McHugh K (2009) Imaging of the unusual pediatric “blastomas”. Cancer Imaging 9:1–11
Perez Castillo A, Aguilar-Morante D, Morales-Garcia JA, Dorado J (2008) Cancer stem cells and brain tumors. Clin Transl Oncol 10:262–267
Peukert S, Miller-Moslin K (2010) Small-molecule inhibitors of the Hedgehog signaling pathways as cancer therapeutics. ChemMedChem 5(4):500–512
Pizer B, Clifford S (2008) Medulloblastoma: new insights into biology and treatment. Arch Dis Child Educ Pract Ed 93(5):137–144
Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G et al (2010) Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J 4;29(15):2646–2658
Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. (2008) Medulloblastomas: from molecular pathology to therapy. Clin Cancer Res 15;14(4):971–976
Saran A (2009) Medullablastoma: Role of developmental pathways, DNA repair signaling, and other players. Curr Mol Med 9(9):1046–1057
Schecterson LC, Bothwell M (2010) Neurotrophin receptors: old friends with new partners. Dev Neurobiol 70(5):332–338
Schmidt AL, de Farias CB, Abujamra AL, Kapczinski F, Schwartsmann G, Brunetto AL, Roesler R (2010) BDNF and PDE4, but not the GRPR, regulate viability of human medulloblastoma cells. J Mol Neurosci 40(3):303–310
Shu X-Q, Mendell LM (1999) Neurotrophins and hyperalgesia. Proc Natl Acad Sci U S A 96(14):7693–7696
Singh SK, Clarke ID, Hide T, Dirks PB (2004) Cancer stem cells in nervous system tumors. Oncogene 23:7267–7273
Skaper SD (2008) The biology of neurotrophins, signalling pathways and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets 7(1):46–62
Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E (2000) Down regulation of the neurotrophin receptor TrkB following ligand binding. J Biol Chem 275(12):8982–8990
Spiller SE, Ditzler SH, Pullar BJ, Olson JM (2008) Response of preclinical medulloblastoma models to combination therapy with 13-cis retinoic acid and suberoylanilide hydroxamic acid (SAHA). J Neurooncol 87(2):133–141
Tanori M, Mancuso M, Pasquali E, Leonardi S, Rebessi S, Di Majo V et al (2008) PARP-1 cooperates with Ptc1 to suppress medulloblastoma and basal cell carcinoma. Carcinogenesis 129(10):1911–1919
Teglund S, Toftgard R (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 1805(2):181–208
Trosko JE (2003) The role of stem cells and gap junctional intercellular communication in carcinogenesis. J Biochem Mol Biol 36(1):43–48
Wlodarski PK, Jozwiak J (2008) Therapeutic targets for medulloblastoma. Expert Opin Ther Targets 12(4):449–461
Wlodarski PK, Boszczyk A, Grajkowska W, Roszkowski M, Jozwiak J (2008) Implication of active Erk in the classic type of human medulloblastoma. Folia Neuropathol 46(2):117–122
Yazigi-Rivard L, Masserot C, Lachenaud J, Diebold-Pressac I, Aprahamian A, Avran D, Doz F (2008) Childhood medulloblastoma. Arch Pediatr 15(12):1794–1804
Yuan L, Santi M, Rushing EJ, Cornelison R, MacDonald TJ (2010) ERK activation of p21 activated kinase-1 (Pak 1) is critical for medulloblastoma cell migration. Clin Exp Metastasis 27(7):481–491
Yue S, Chen Y, Cheng SY (2009) Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin-proteasome pathway. Oncogene 28:492–499
Zavarella S, Nakada M, Belverud S et al (2009) Role of Rac 1-regulated signaling in medulloblastoma invasion. J Neurosurg Pediatr 4(2):97–104
Zitterbart K, Filkova H, Tomasikova L, Necesalova E, Zambo I, Kantorova D et al (2010) Low-level copy number changes of MYC genes have a prognostic impact in medulloblastoma. J Neurooncol 102(1):25–33
Acknowledgments
This article has been prepared and devoted to the memory of Veysel Yüksekol who has lost his life from adult medulloblastoma. We are grateful to Ernest Jennings for his valuable comments and language support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sümer-Turanlıgil, N.C., Çetin, E.Ö. & Uyanıkgil, Y. A contemporary review of molecular candidates for the development and treatment of childhood medulloblastoma. Childs Nerv Syst 29, 381–388 (2013). https://doi.org/10.1007/s00381-012-2014-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-2014-3