Abstract
Background
Multiple neural tube defects (MNTDs) are a rare occurrence. Although the clinical incidence is small, MNTDs raise some interesting embryological queries.
Aim
This study aims to investigate the morphological and clinical variations observed in neonates presenting with multiple neural tube defects and associated central nervous system anomalies.
Materials and methods
This is a prospective study carried out at our institute to assess clinical and morphological variation in patients presenting with multiple neural tube defects.
Results
Among the 263 patients with a neural tube defect, who presented to our outpatient department and emergency departments, only 10 cases of MNTDs were identified. Thus, incidence of MNTDs in the cohort of patients affected with NTD was 0.038 %. Among the 10 patients, 9 had double neural tube defects and 1 patient had three neural tube defects.
Conclusions
Multiple neural tube defects may not be very rare in the general population, especially the populations with high incidence of neural tube defects. Multisite closure theory has the versatility to explain various combinations of neural tube defects, but better insights into the molecular pathways governing this tightly regulated process can provide us the missing link in establishing the pathogenesis of multiple NTDs. It can also provide us with an opportunity to prevent NTDs or treat them in utero by pharmacological modulation of these signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural tube defects (NTDs) are one of the common birth defects encountered in the pediatric population. They are the direct consequences of aberrations of neurulation, which is one of the most complex morphogenetic events in embryogenesis. Although single NTD is most commonly seen, multiple NTDs in a single patient are extremely rare. The complexity of the process has been explained by multiple theories based on different investigational observations. Multisite closure theory comes very close to explain multiple NTDs in a single patient.
Only a few cases of multiple NTDs are reported in the world literature comprising about 20 cases of double NTDs and only 4 cases of triple NTDs. Meningomyeloceles are defects in primary neurulation, while lipomeningomyeloceles below the sacral levels are defects of secondary neurulation. Encephalocele represents a malformation of the skull vault following closure of the neural tube, and it is not a primary anomaly of neural tube closure. It could, however, arise as a secondary consequence of abnormal signaling from an inappropriately patterned neural tube.
This study aims to investigate the morphological and clinical variations in children with multiple NTDs. We have further tried to rationalize our observations in light of the molecular evidences gathered over the years. These molecular evidences stem from various animal experiments, and we believe that a rational correlation with phenotypes seen in human subjects may prove useful.
Materials and methods
This is a prospective study carried out at our institute between November 2009 and August 2011. All cases of neural tube defects that were seen in our outpatient department (OPD) were examined carefully for the presence of multiple neural tube defects (MNTDs). The patients underwent thorough clinical examination, including the number and type of defect, associated hydrocephalus, sensory and motor impairment in lower limbs along with bowel and bladder involvement. The presence of meningomyeloceles (MMCs) at multiple sites (two or more) or their association with an encephalocele was formulated as a working definition of MNTDs. Patients with obvious swelling in the midline from occipital region to the sacral region or a cutaneous stigma suggestive of an underlying occult NTD were actively sought for and investigated. The patients were investigated with relevant radiological investigations to document the location, exact anatomy of the defect, and any associated malformations. Depending on the site of neural tube defects, the closure site was estimated according to the multisite closure theory.
Results
In a time span of 22 months, OPD attendance amounted to 37,202 patients. Of these, 263 patients were diagnosed to have neural tube defects. Among the 263 patients who had a neural tube defect, only 10 cases of MNTDs were identified. Thus, incidence of MNTD in the cohort of patients affected with NTD was 0.038 %. The age group at presentation ranged from 1 day to 9 months with a median age of 12 days. There were six male and four female children. Among the 10 patients, 9 had double neural tube defects and 1 patient had three neural tube defects.
Case 1
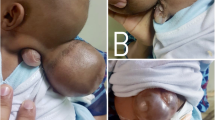
A 12-day-old female child presented with a swelling in the posterior aspect of head and lower back since birth. On examination, there was a 7 × 7 cm, pedunculated swelling in the occipital region, which was cystic in consistency and transilluminant. The second swelling was in the lumbar region, of size 3 × 3 cm, soft in consistency, covered with skin, and with a palpable underlying bony defect (Fig. 1a). No neurological weakness was evident in the lower limbs. Computed tomography (CT) scan of cranium revealed a large occipital encephalocele containing cerebrospinal fluid (CSF), meninges, and brain parenchyma with a defect of 1.5 cm in the occipital bone. There was no evidence of hydrocephalus. The child was operated for occipital encephalocele in the neonatal age. On follow-up at 8 months of age, magnetic resonance imaging (MRI) of spine revealed a lumbar lipo-meningomyelocele (lipo-MMC) at the L4–L5 level. Presently, the child is awaiting surgery for lipo-MMC.
Case 2
A nine-month-old male child presented with two midline swellings in the back since birth. On examination, a 2 × 2 cm, skin-covered cystic swelling was noted at the mid-thoracic level with underlying bony defect. Another swelling of 8 × 7 cm size with similar characteristics was noted in the lumbar region (Fig. 1b). The anterior fontanelle (AF) was flat, and the child had bilateral lower limb flaccid paralysis with patulous anus and fecal incontinence. After counseling and thorough discussion about the management and prognosis, the parents denied any further investigations and treatment in any form.
Case 3
A two-month-old male child presented with multiple swellings on the back, in the midline, since birth. On examination, the AF was open but flat. The child had three swellings on the back, one extending from the lower cervical to the upper thoracic region measuring 4 × 4 cm in size. It was lobulated, soft, and cystic covered with a thin membrane. Another swelling of the same size and consistency covered with thinned out skin was present at lower thoracic and upper lumbar region. There was a third swelling measuring 5 × 5 cm, soft in consistency covered with normal healthy skin with an overlying dimple observed at the sacral region. The child had no apparent bilateral lower limb motor weakness. The anal muscle tone appeared normal, and the urinary bladder was not palpable on abdominal examination.
MRI of the head and entire spine showed dilated lateral and third ventricular system with a hypoplastic single-lobed cerebellum with absent vermis and compressed fourth ventricle, suggestive of rhomboencephalosynapsis with cerebellar herniation through the foramen magnum. The spinal cord was seen to herniate through a defect at C5 to T1 level, and CSF out pouching was also seen at the T10 to L2 level with a focal syrinx. The low lying spinal cord terminated at the level of L5 vertebra and was tethered. Another spinal defect was also seen involving the sacral vertebra with herniation of CSF, meninges, and neural placode (Fig. 2).
The child initially underwent a ventriculoperitoneal shunt followed by repair of cervical meningomyelocele and dorsal meningocele at a second sitting. The cervical cord was untethered. The swelling at the dorsolumbar level was a meningocele without any prolapse of neural tissue or tethering. There was a skin defect after operating at the dorsolumbar level, which required a plastic surgical intervention; hence, it was decided to repair the sacral lipo-MMC at a later date.
Case 4
A newborn male child presented with multiple swellings over back in midline. On examination, there was a bilobed cystic swelling, which was translucent, present in the lower thoracic region with another swelling of 6 × 6 cm size in the lumbar region, which was covered with skin and soft in consistency (Fig. 3). The AF was full and bulging. The child had bilateral lower limb weakness (right side power, 1/5; left side power, 2/5) with patulous anal opening. Ultrasonography of cranium revealed hydrocephalus with ventriculohemispheric ratio (VHR) of 70 %, with a special mention of small posterior fossa and absent corpus callosum. The parents were counseled about the management and prognosis. MRI of brain and spine was advised, but the child was lost to follow-up.
Case 5
A four-day-old female child born at full term by normal vaginal delivery presented to us with two swellings on the back since birth. There were two swellings: one in the thoracic region of 7 cm diameter, soft in consistency and covered with normal healthy skin. Another swelling of 3 × 4 cm size soft and cystic in consistency, transilluminant, and covered with partially thinned out skin was present in the lumbo-sacral region. The child had sensorimotor deficit of bilateral lower limbs with power in each limb of 2/5. The AF was flat. MRI spine revealed diffuse kyphosis of dorsal spine and straightening of lumbosacral spine. There was evidence of myelomeningocele with lipomatous component at L2–L5 level. There was formation of a syrinx at T9–T10 and at T11 level; there was focal herniation of meningeal sac and spinal cord with tethering, suggestive of closed meningomyelocele. There was presence of hydrocephalus and tonsillar herniation up to C4 level suggestive of type II Arnold–Chiari malformation (Fig. 4). When explained about the long-term prognosis and outcome, parents denied for any form of treatment.
Case 6
An eighteen-day male child born at full term by normal vaginal delivery presented with a small swelling in the lumbar region. He was antenatally detected to have split cord malformation at the lumbar region on fetal ultrasonography (USG) and confirmed by fetal MRI at 33 weeks of gestation. Fetal MRI revealed low lying cord with tethering and myelocele in that region. On postnatal examination, the AF was supple with no sensorimotor deficit in the lower limbs. There was a 1.5 × 1.5 cm, skin-covered swelling with a tuft of hair in the lumbosacral region. Approximately 5 cm below it, another swelling with a bony spur was present with a dermal sinus overlying it. Postnatal MRI revealed a meningocele with a dorsal dermal sinus at L5 vertebra with type I split cord malformation (SCM. There was evidence of syrinx in the cord at L2–L3 level with a well-defined 2.3 × 1.2 cm epidermoid cyst in the whole of sacral canal (Fig. 5). The brain parenchyma and posterior fossa structures were normal with no evidence of hydrocephalus. The patient underwent repair of meningocele and excision of epidermoid cyst at the Department of Neurosurgery.
a Clinical photograph showing lumbar meningocele with tuft of hair. b Magnetic resonance image (MRI) (sagittal view) depicting associated split cord malformation (red arrow) and meningocele (yellow arrow). c MRI (axial view) showing meningocele (yellow arrow). d MRI (axial view) showing split cord malformation (red arrow)
Case 7
About a month old female child born at full term by a lower segment caesarean section presented with a swelling in the back of the head. She was antenatally diagnosed to have an occipital encephalocele on a routine level II ultrasound. On examination, there was a large 8 × 8 cm swelling, soft cystic in consistency covered with normal healthy skin in the occipital region. Just adjacent to it, another swelling of similar nature but smaller in size (3 × 3 cm) was present. There was an elliptical desquamated area at the lumbar region without any obvious underlying bony defect. The AF was flat, and there was no sensorimotor deficit in all four limbs. MRI revealed occipital encephalocele with bony defect in the occipito-parietal bone with herniation of both occipital and parietal lobes through the defect (Fig. 6). A note was also made of shallow posterior fossa and a tonsillar herniation. There was associated hydrosyrinx from the C5 to T8 and T11 to L1 region. The child underwent excision and repair of the occipital encephalocele.
Case 8
A three-month-old male child born at term by normal vaginal delivery to primigravida mother presented with a swelling on the back of the head since birth. On examination, the child appeared to thrive well, with open and flat AF. . Approximately 7 × 6 cm pedunculated soft cystic swelling arising from suboccipital region and a 3 × 3 cm discolored patch with abnormal hair growth were noted in the lumbar region with underlying spinal defect (palpable). There was no sensorimotor deficit of both the lower and upper limbs. On MRI, a 7 × 5 cm × 4 cm large suboccipital meningocele connected to the spinal canal at the craniovertebral junction with a fibrous band in the lesion was noted. There was no other intracranial anomaly. Diffuse syrinx was seen in the cervical and dorsal spinal cord with evidence of spina bifida occulta at the L3, L4, and L5 vertebral levels (Fig. 7). The child underwent repair of the suboccipital meningocele.
Case 9
A two-day-old male child born by normal vaginal delivery to second gravida mother presented with two swelling on the back since birth. On examination, there was a 3 × 3 cm soft cystic swelling in the cervical region with thin sac and another large lobulated swelling of 6 × 7 cm was present in the lower thoracic region with thin skin and big underlying vertebral defect without any CSF leak. There was motor weakness on left lower limb (power grade, 3/5) and no motor weakness of right lower and bilateral upper limbs; the AF was bulging. On USG cranium, ventricular system was dilated with VHR approximately 50 %, septum pellucidum was not visualized; suspicion of aqueductal stenosis was present. The child underwent right VP shunt for hydrocephalus. Later, MRI of brain was done, which revealed features of Arnold–Chiari malformation type III with low occipital encephalocele communicating to posterior fossa with an osseous defect in basiocciput. Additionally, the cerebellar hemispheres were dysplastic, and there was downward herniation of cerebellar tonsil and markedly thin, hypoplastic to partially absent corpus callosum. MRI of spine showed presence of multisegmental meningocele from T8–T9 to L1 region with multisegmental thin syrinx from T3–T4 to T8–T9 (Fig. 8).
Case 10
A five-day-old female child born to a third gravida mother by normal vaginal delivery presented with two swellings on the midback with tufts of hair arising from the sides. On examination, there were two obvious swellings on the back. A. swelling of size 4 × 5 cm soft cystic consistency with thin overlying skin was noted at the lower thoracic vertebral region without any CSF leak. Another swelling was noted just below it in the midline of size 4 × 4 cm, soft in consistency, and was covered with normal skin. Tuft of hair was noted arising from the lateral side of the swellings (Fig. 9). The child’s anterior fontanelle was flat, and there was no sensorimotor deficit in the lower limbs noted. A clinical diagnosis of thoracic MMC and lumbar lipo-MMC was made, and it was supported by findings at USG. This child was lost to follow-up and hence could not be investigated further with MRI.
Discussion
While analyzing the cases of MNTDs, we concluded that a triple neural tube defect was observed in one case (0.004 %) and the rest of the nine cases (0.034 %) had double neural tube defects. Various combinations of meningomyeloceles, lipomeningomyeloceles, encephelaloceles, and dermal sinus were seen as enumerated in Tables 1 and 2. A specific combination of defects was not observed in our series. Similar findings have been reported by Ahmad et al. [1] in their maiden retrospective series. Although our clinical findings are in line with the observations made by Ahmad et al., our prospective series highlights certain differences.
The time period of our study was nearly about 2 years, whereas the study period in prior series was about 5 years. Despite a shorter study period, we did see an equal number of patients with this rare anomaly. We believe that this difference is seen because of the prospective nature of our study and a proactive approach to search for cases of MNTD among the patients of NTD. Thus, it seems likely that multiple neural tube defects might be commoner in the general population, especially the populations with high incidence of NTDs.
We also noted varying combinations of cervical, thoracic, and lumbar defects in our series, but no consistent combination could be seen. The length of the defects observed varied to a large extent. Associated neural tube anomalies, distinct from the clinical neural tube defects, were observed in seven cases. We could not ascertain the presence of associated central nervous system (CNS) anomalies in two cases as the MRI was denied by the parents. Only one patient did not have any associated CNS anomalies on MRI. It symbolizes that neural tube defect may not be just a localized disruption of neural tube closure. The whole neural tube might be developmentally affected, and segmental open defects only represent a more severe presentation of the underlying problem. Although encephalocoeles are post-neurulation defects and lumbar lipoMMCs are mostly secondary neurulation defects, they are usually grouped into a common clinical spectrum of neural tube defects. The clinical sequelae of lipo-MMC are also similar, though late.
Four of our patients had associated hydrocephalus, three had lower limb weakness, and three patients had patulous anus. All patients who had a lower limb weakness had a patulous anus. All these patients had a lumbar MMC. Our findings represent a marked clinical heterogeneity associated with phenotypic heterogeneity, which is a hallmark of these anomalies. The observed heterogeneity can be explained on the basis of a multitude of downstream regulatory processes, which govern the closure of a neural tube. It is thus important to go beyond the traditional neural tube closure theories and closely study these regulatory processes. A better understanding of molecular signaling involved in this process may help us in developing preventive strategies.
Theories to explain neural tube defects
Multiple theories have been described to explain the complex event of neural tube closure. No theory has conclusively been accepted or refuted, and hence, the matter seems to be under constant scrutiny.
According to the traditional primary closure theory, the “zipper” proceeds rostrally and caudally to close the neural tube. In the early 1990s, with the evidence obtained from animal studies, Van Allen et al. [10] contested this single site initiation theory and proposed the multisite closure model of the neural tube in human embryos. They proposed that there are five sites of initiation of neural fold fusion. Although multiple sites of initiation were described, the exact meeting points were not discussed. In 2000, Nakatsu et al. [5] studied 68 normal embryos and observed three sites of initiation of closure of neural tube. while O’Rahilly and Muller [6] observed only two sites of initiation of fusion of neural folds. They observed that closures 4 and 5 as referred by Van Allen et al. do not actually exist in either mouse or human embryos. On the other hand, Srinivas et al. [8], while reporting the two cases of triple neural tube defects, raised a question of presence of an additional site (site 6) [8]. Thus, the number of initiation sites of neural tube closure still remains a matter of speculation.
We examined whether the observed neural tube defects could be explained on the basis of these multisite closure theories. Two different phenomena, namely, (1) the total lack of a closure point and (2) the result of faulty propagation of neurulation away from a closure point, seem to be responsible for different neural tube defects. Hence, total failure of closure 1 yields craniorachischisis (not seen in any of our cases), whereas failure of neurulation to propagate caudally from closure 1 generates myelomeningocele, as seen in six of the cases.
These observations leave us with a very fascinating question: How can the multiple NTDs along the spinal region can be explained? Although multisite closure theory envisages the formation of multiple initiation sites, it banks upon caudal and rostral “zippering” to explain the process of neural tube closure. However, it falls short of explaining the genesis of segmental and/or multiple neural tube defects. The only plausible explanation for segmental neural tube defects is that this “zippering” process tends to start once again even if interrupted [7]. The zipper starts again means a new (rescue) initiation site is formed. With this argument, we take a fresh look at this theory from a different perspective.
Theory to explain MNTDs
The concept of neural tube closure proceeding rostrally and caudally from the initiation site(s), as explained by the multisite closure theory, is rather simplistic. The closure “proceeds” conversely means that the subsequent segment lags behind the preceding segment temporally. It also means that the closure of one segment is a prerequisite for subsequent segments to close.
In our view, each segment of the neural tube closes independent of its adjacent segments under the influence of the subjacent notochordal segment. This phenomenon is akin to the “Mexican Wave” where the events are linked temporally but are actually independent of one another, contrary to a “domino effect,” where occurrence of one event leads to that of another event.
The complex interplay of Shh and its antagonist, Wnt, has been shown to be prime mediator for initiation and sustenance of neural tube closure. Shh inhibits dorsolateral hinge point formation, and Wnt, in turn, represses the Shh activity [2, 9]. This mechanism operates at all levels of the body axis, during neurulation [3]. The strength of Shh signaling is higher in the rostral than in the caudal regions. Furthermore, there is evidence that there is also a temporal decline in this strength from rostral to caudal direction as “the wave of neurulation progresses.” This intriguing spatial and temporal decline can be explained in following ways:
-
1.
Some other morphogens other than Shh act in concert with Shh, and their additive effects are achieved in the succeeding segment with a delay.
-
2.
The cell responsiveness is achieved in the preceding segment earlier, and hence, that segment closes earlier.
Experiments have shown that focal alterations in strength of Shh signaling lead to focal disruption of neural tube closure with normal neural tube closure in the flanking segments. Above observations make us believe that every segment has an independent potential to close, thanks to the local regulatory factors. We suggest that the initiation site is merely that segment of the neural tube that closes first. Each subsequent segment independently closes as the gradient of the pro- and anti-closure factors is attained chronologically with the wave of neurulation. This forms the basis for the apparent “zippering.”
A brief/local insult of these regulatory pathways may not affect the ability of succeeding segments to close. The longevity and local extent of the insult shall govern the extent of NTD. Once the incriminating insult dies out, the subsequent segments close due to their inherent potential.
Such multiple disruptions because of multiple but transient environmental insults (e.g. maternal fever) can explain the pathogenesis of multiple NTDs [4].
Conclusions
We strongly believe that multiple neural tube defects might be commoner in the general population, especially the populations with high incidence of NTDs. This warrants a proactive approach to search for concomitant neural tube anomalies in each and every patient of neural tube defect.
Inspite of the versatility of the theory in explaining various combinations of neural tube defects, the theory stops short of elucidating the exact mechanisms involved in specification of the initiation sites and sequential closure of the neural tube. Better insights into the molecular pathways governing this tightly regulated and exquisitely well-choreographed process can provide us the missing link in establishing the pathogenesis of multiple NTDs. It can also provide us with an opportunity of preventing or treating NTDs in utero by pharmacological modulation of these signaling pathways.
References
Ahmad FU, Dwarakanath S, Sharma BS et al (2008) Multiple neural tube defects: a clinical series of seven cases and their embryological basis. Pediatr Neurosurg 44:280–287
Copp AJ, Greene NDE (2010) Genetics and development of neural tube defects. J Pathol 220:217–230
Copp AJ, Greene NDE, Murdoch JN (2003) The genetic basis of mammalian neurulation. Nat Rev 4:784–793
Mahalik SK, Vaze D, Lyngdoh TS et al (2011) Embryogenesis of triple neural tube defects: sonic hedgehog—a key? J Ped Surg 46:E5–E8
Nakatsu T, Uwabe C, Shiota K (2000) Neural tube closure in human starts at multiple sites: evidence from human embryos and implications for the pathogenesis of neural tube defects. Anat embryo (Berl) 201:455–456
O’Rahilly R, Muller F (2002) The two site of fusion of the neural folds and the two neuropores in the human embryo. Teratology 65:162–170
Pang D (2010) Commentary to the paper double neural tube defect: a case report and discussions on neural tube development by V. Ravindran. Childs Nerv Syst 26:703
Srinivas D, Sharma BS, Mahapatra AK (2008) Triple neural tube defect and the multisite closure theory for neural tube defects: is there an additional site? J Neurosurg Pediatr 1:160–163
Ulloa F, Martı E (2010) Wnt won the war: antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube. Dev Dyn 239:69–76
Van Allen MI, Kalousek DK, Chernoff GE et al (1993) Evidence for multi-site closure of the neural tube in humans. Am J Med Genet 47:723
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahalik, S.K., Vaze, D., Kanojia, R.P. et al. Multiple neural tube defects may not be very rare. Childs Nerv Syst 29, 609–619 (2013). https://doi.org/10.1007/s00381-012-1976-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-1976-5