Abstract
Purpose
The purpose of this study is to describe the technique and advantages and limitations of spring-assisted cranioplasty for sagittal suture synostosis.
Methods
Preliminary data are presented of the first 41 patients treated with this technique at our institution.
Results
The cephalic index was 75 after surgery and dropped to 74 one year after surgery. Mean blood loss of both procedures combined was 54 ml.
Conclusion
Spring-assisted cranioplasty requires only two small incisions and is at least as effective as other techniques with respect to the cephalic index. Blood loss, operative time, and complication rate are reduced. The most important disadvantage is the need to remove the springs in a second intervention. A second drawback is that the expansion of the spring is not controllable after placement. This can be partially intercepted by adjusting the spring (or the craniotomy) to the patient’s specific features.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous techniques have been described to correct the scaphocephalic head shape that results from early closure of the sagittal suture. Procedures range from total cranial remodeling to helmet-assisted endoscopic strip craniectomy. Arguments for minimally invasive procedures are reduced operation time and reduced blood loss. Arguments for total cranial remodeling are a durable cosmetic result and sufficient cranial volume. At the Dutch Craniofacial Center, we used different techniques and found that extended strip craniectomy indeed had a shorter operating time and reduced blood loss, but also showed a faster decrease in cranial volume compared to total cranial remodeling. The ideal cranial volume after surgery for scaphocephaly is not known. However, the percentage of patients showing signs of raised intracranial pressure after extended strip craniectomy was higher than expected, which led us to hypothesize that the achieved cranial volume with our technique of out-fracturing the parietal flaps and removal of the sagittal strip was not sufficient.
Therefore, we changed to a minimally invasive procedure combined with springs. The distractive forces of the springs not only correct shape but might also create extra cranial volume.
Spring-assisted correction of sagittal suture synostosis was introduced by Lauritzen et al., who described the first case in 1998 [3]. The technique generally consists of a strip craniectomy or linear craniotomy followed by placement of a series of internal springs perpendicular to the synostosed suture. The distractive force of the springs gradually remodels the skull. After a period of re-ossification, the springs need to be removed. Nowadays, more than a decade after its introduction, this technique is becoming more widely adopted [1, 7]. At the Dutch Craniofacial Center, springs were introduced in 2010. Since then, 65 patients underwent spring-assisted correction. Of these 65 patients, 44 had synostosis of the sagittal suture. We describe the technique and preliminary results of this procedure.
Preferred surgical technique
The patient is positioned on his left side. This position offers better access than the supine position and is safer than the prone or sfinx position. Photographs of the technique are shown in Fig. 1. Two 4-cm-wide skin incisions are placed perpendicular to the sagittal suture, 3–4 cm behind the coronal suture and 3–4 cm in front of the lambdoid suture and 6–7 cm apart. The galeal plane is dissected along the sagittal suture. Two periostal incisions are placed parallel to and at 0.5–1 cm distance from the sagittal suture, running from coronal to lambdoid suture. At the level of the skin incision, two burr holes are made, left and right from the sagittal suture. Starting from these burr holes, a craniotomy is performed going forwards and backwards as far as the skin incision permits, and completing the craniotomy line in the middle and all the way to the coronal suture and lambdoid suture using scissors. The strip can be left in place. Finally, two large springs (see below for the choice of springs) are placed over the strip in the indentations that result from the burr holes. The skin is closed.
After 8–12 weeks, the springs can be removed through the same incisions. The spring needs to be cut with a k-wire cutter as close to the lateral ends as possible. The lateral ends can be grabbed with a needle holder and removed with a rotating movement that follows the foot of the spring.
Techniques described in the literature
Incision
Descriptions of the spring-assisted technique by Lauritzen et al. [4] and David et al. [1] mention the lazy S incision. This incision gives an excellent overview over the sagittal suture. If chosen correctly, the scar will be hidden by hair. However, because the distractive forces are perpendicular to the incision, the incision tends to widen, especially at the parts where the incision runs parallel to the suture. The small incisions we use are placed perpendicular to the distractive forces and remain narrow after correction of the skull. With some experience, and use of adequate elevators and a headlight or facelift endoscope, preparing and performing the craniotomy line is feasible. The approach by two small incisions is described in numerous articles for the endoscopic-assisted technique. We found that without an endoscope, but with a good headlight, the overview is sufficient. Also, Taylor et al. report that the use of an endoscope is unnecessary [7].
Choosing type and location of springs
Lauritzen et al. and Windh et al. use hand-bent springs from stainless steel wire [4, 8]. The spring is 1.2 mm thick and 16 cm long with a distraction force between 6 and 8 N at an interarm distance of 10–15 mm. They perform a midline craniotomy. The springs are placed in two drilled holes at 1–2 cm apart on the left and right side of the craniotomy, posterior to the coronal suture and anterior to the lambdoid suture.
David et al. use the same technique but remove a 1-cm wide strip along the sagittal suture [1]. The springs are manufactured before surgery based on a raisin model of the 3D CT scan of the patient. Length and thickness of the wire and diameter of the bending curve are chosen based on the particular features of the patient. The wire (SS316 V) they use is mostly 1.28 mm thick, and the spring generally has a distraction force of between 5.5 and 9.5 N. An extensive description of the manufacture and utilization of springs is given by Pyle et al. [6].
Taylor et al. remove a 2-cm-wide strip. They place three springs with forces ranging from 5–7 N in ascending force from anterior to posterior [7].
We decided to use standard springs manufactured by the Active Spring Company (Fig. 2). These springs have a helix, which enhances the durability of the extensive forces. They are produced in two sizes. The small ones are 6 cm wide before placement and have a distractive force of 6 N. The larger ones are 8.9 cm wide and have 9 N of distractive force. The arms of the spring are slightly bent to follow the curvature of the skull. The wire is from medical steel, BS 2056, grade 316S42 (1.22 mm thick) and manufactured and tested by the Active Spring Company (Sibleys Green, Thaxted, Essex, UK).
Hand-bent springs have the advantage that they can be adjusted to the particular features and age of the patient. However, standard springs are easy and reliable. Most patients present early, and they can be planned to undergo surgery at 5–6 months of age. Instead of adapting the spring, the width of the strip or the position of the troughs for the footplates of the wire can be varied according to the distraction that is required.
Timing
The age of the patients operated by Lauritzen et al. ranged from 2.5 to 8 months [3]. The mean age at surgery in studies on spring-assisted cranioplasty ranges from 3.5 to 5.7 months [1, 4, 7, 8].
Patient care
As soon as the patient presents we advise back positioning of the head. Following this simple advice reduces the occipital bullet in most patients, while the child becomes accustomed to the ideal position after placement of the springs.
Some patients may experience pain directly after placement of the springs, probably related to the high rate of expansion at the beginning. This can be resolved with paracetamol in most cases.
Parents should be informed about the occurrence of prominent ridges at the level of the coronal and lambdoid suture. Those ridges illustrate the effect of the springs and will subside slowly in the year after placement. Springs can be removed 8–12 weeks after placement.
Preliminary results
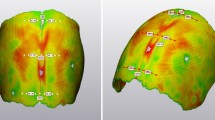
Patients with scaphocephaly who presented to our institution before the age of 6 months were selected to undergo spring-assisted correction. We collected data on age, blood loss, and cranial index of a consecutive series of 41 patients operated between January 2010 and January 2012. Patients were operated at a mean age of 5.8 (range, 3.5–6.5) months. Preoperative cranial index was 66.8 (range, 56–74; SD 4.4), and postoperative cranial index at removal of the springs was 75.4 (range, 68–85; SD 4.0). This increase was statically significant (paired samples t-test, p = 0.003). Cranial index at 1 year after surgery is only available for a smaller subset of 20 patients and shows a decrease to 73.7 (range, 63–79; SD 4.7). Not only does the cranial index improve but also the cranial height, as shown in Fig. 3. Mean blood loss was 53.8 ml (range, 20–150; SD 20.1).
The first patient was undercorrected, but the parents were satisfied with the result. We observed no spring displacements or skin perforations. In one patient, leakage of clear fluid was observed immediately after removal of the spring. The opening was sealed with bone wax. The patient received a compressive draping for 5 days. At follow-up, no signs of persevering CSF leakage were found, nor any swelling or growing skull defects.
Discussion
The results in term of cranial index are comparable to our earlier results with other techniques, and also with results reported in the literature [1, 3, 7, 8]. In these latter studies, postoperative cranial index ranges from 0.72 to 0.79. Windh et al.report a slight relapse at 3 years, while David et al. show that cranial index is maintained at 3–5 years after surgery [1, 8]. Follow-up in our series is too short to evaluate the occurrence of signs of raised intracranial pressure after the spring-assisted cranioplasty.
Spring-mediated cranioplasty has been compared to other techniques, mainly to historical series from the same institution. Windh et al. compared spring-assisted cranioplasty to the pi-plasty, David et al. to cranial vault remodeling and Taylor et al. to minimally invasive strip craniectomy with barrel staving [1, 7, 8]. In all studies, surgical time and blood loss were significantly lower for the spring-assisted cranioplasty, while the postoperative cranial index was higher or comparable.
Complications reported in the literature occurred only occasionally and consisted of undercorrection, local skin infection, scar revision, and spring displacement. Advantages and limits of this technique are listed in Table 1. One drawback is the fact that once the spring is placed, the amount of distraction cannot be influenced. Carefully choosing the spring and the position of the troughs can avoid suboptimal distraction.
Another obvious drawback is the need to remove the springs in a second intervention, even if this intervention is short, well tolerated, and performed in day care. The alternatives to achieve remodeling after minimal incisions are either to add barrel staves and rely on positioning on the back or to give a helmet [2, 5]. However, both may fail to create sufficient cranial volume. Minimally invasive procedures are of course preferable, but not at the expense of remodeling and sufficient cranial volume.
Conclusion
Spring-assisted cranioplasty can be performed via a minimal incision. Surgical duration and mean blood loss are significantly reduced compared to other techniques. Complications are limited. Cosmetic results seem to be comparable to more extensive techniques. More extensive follow-up is needed to better evaluate the sustainability of the results.
References
David L, Plikatis CM, Couture D et al (2010) Outcome analysis of our first 75 spring-assisted surgeries for scaphocephaly. J Craniofac Surg 21:3–9
Jimenez DF, Barone C, McGee ME et al (2004) Endoscopy-assisted wide vertex craniectomy, barrel stave osteotomies, and postoperative helmet molding therapy in the management of sagittal suture craniosynostosis. J Neurosurg Pediatr 100:407–417
Lauritzen C, Sugawara Y, Kocabalkan O et al (1998) Spring-mediated dynamic craniofacial reshaping. Scand J Plast Reconstr Hand Surg 32:331–338
Lauritzen CGK, Davis C, Ivarsson A et al (2008) The evolving role of springs in craniofacial surgery: the first 100 clinical cases. Plast Reconstr Surg 121:545–554
Mutchnick IS, Maugans TA (2012) Nonendoscopic, minimally invasive calvarial vault remodeling without postoperative helmeting for sagittal synostosis. J Neurosurg Pediatr 9:222–227
Pyle J, Glazier S, Couture D et al (2009) Spring-assisted surgery—a surgeon’s manual for the manufacture and utilization of springs in craniofacial surgery. J Craniofac Surg 20:1962–1968
Taylor JA, Maugans TA (2011) Comparison of spring-mediated cranioplasty to minimally invasive strip craniectomy and barrel staving for early treatment of sagittal craniosynostosis. J Craniofac Surg 22:1225–1229
Windh P, Davis C, Sanger C et al (2008) Spring-assisted cranioplasty vs Pi-plasty for sagittal synostosis—a long-term follow-up study. J Craniofac Surg 19:59–64
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was not subject to the Medical Research Involving Human Subjects Act (WMO) since this study does not involve any form of invasion of the study participant's integrity.
Rights and permissions
About this article
Cite this article
van Veelen, ML.C., Mathijssen, I.M.J. Spring-assisted correction of sagittal suture synostosis. Childs Nerv Syst 28, 1347–1351 (2012). https://doi.org/10.1007/s00381-012-1850-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-1850-5