Abstract
There have been significant recent advances in the past several years in the field of neurocutaneous vascular syndromes, including the development of more stringent diagnostic criteria for PHACE syndrome, the renaming of macrocephaly-cutis marmorata telangiectatica congenita to macrocephaly-capillary malformation to accurately reflect the true nature of the syndrome, and discovery of new genetic mutations such as RASA-1. There have also been advances in the understanding and management of Sturge-Weber syndrome.
PHACE syndrome is a constellation of neurologic, arterial, cardiac, ophthalmologic, and sternal abnormalities associated with infantile hemangiomas. PHACE is an acronym for Posterior fossa malformation, Hemangioma, Arterial anomalies, Coarctation of the aorta, Eye abnormalities. Some authors include an “S” for PHACE(S) to denote the association of ventral defects including Sternal clefting and Supraumbilical raphe.
The accurate diagnosis and work-up of these patients require coordination of care across multiple disciplines, including neuroradiology, radiology, dermatology, neurology, surgery, and interventional radiology, among others.
This paper is meant to update clinicians and researchers about important advances in these rare, important vascular syndromes, to improve care, and lay the foundation for future directions for research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There have been significant recent advances in the past several years in the field of neurocutaneous vascular syndromes, including the development of more stringent diagnostic criteria for PHACE syndrome, the renaming of macrocephaly-cutis marmorata telangiectatica congenita (M-CMTC) to macrocephaly-capillary malformation (M-CM) to accurately reflect the true nature of the syndrome, and discovery of new genetic mutations such as RASA-1. There have also been advances in the understanding and management of Sturge-Weber syndrome (SWS). The accurate diagnosis and work-up of these patients require coordination of care across multiple disciplines, including neuroradiology, radiology, dermatology, neurology, surgery, and interventional radiology, among others. This paper is meant to update clinicians and researchers about important advances in these rare, important vascular syndromes, to improve care, and lay the foundation for future directions for research.
PHACE syndrome
PHACE syndrome (OMIM 606519) is a neurocutaneous vascular disorder of relatively recent description, which is now thought to be perhaps more common than SWS [32]. First reported under its present moniker by Frieden et al. in 1996 [15], and initially recognized by Pascual-Castroviejo in 1978 [36], PHACE syndrome is a constellation of neurologic, arterial, cardiac, ophthalmologic, and sternal abnormalities associated with infantile hemangiomas (IH). PHACE is an acronym for Posterior fossa malformation, Hemangioma, Arterial anomalies, Coarctation of the aorta, Eye abnormalities. Some authors include an “S” for PHACE(S) to denote the association of ventral defects including Sternal clefting and Supraumbilical raphe. The IHs of PHACE are generally segmental in appearance, following reproducible patterns that appear to arise from developmental units, as opposed to focal IH which seem to develop from a single point. Recent work has elucidated four such reproducible patterns on the face, dubbed Segment 1 (frontotemporal forehead and upper eye with extension to the scalp), Segment 2 (maxillary), Segment 3 (mandibular—or what has been previously described as the “beard” distribution), and Segment 4 (frontonasal, involving a strip of skin from the superior forehead along the nose extending to the philtrum) [21] (Fig. 1). These segments do not follow dermatomes nor do they follow Blaschko’s lines (embryonic cleavage lines that recur in many dermatologic disorders). There are reports of PHACE involving segmental IH of the neck, chest and limbs, but these are much less common, as more than 98% of IH in PHACE occur on the face and scalp [31].
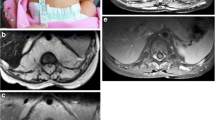
a Infant with typical segmental telangiectatic and reticular appearing infantile hemangioma of PHACE syndrome, primarily involving Segment 1. b Post-Gd T1-weighted image shows lobular, avidly enhancing hemangiomas in the left facial subcutaneous compartment (arrow), and along the eyelid, preseptal, as well as retrobulbar compartments of the left orbit (arrowheads). Persistent embryronic arteries can be seen in PHACE syndrome. In this case, evident on both MIP and axial source images of the head MRA, there is a “true” fetal origin of the right posterior cerebral artery that is fed by the anterior choroidal artery (curved block arrow), while a right posterior communicating artery connects the right internal carotid to the basilar artery (straight block arrow), in a configuration that is seen in fetus
Until recently, the diagnostic criteria have been extremely broad, requiring only the presence of an IH and one extracutaneous manifestation. A consensus statement has been published dividing patients into categories of definite PHACE or possible PHACE syndrome based on major and minor criteria of cerebrovascular, structural brain, cardiovascular, eye, and ventral systems. To diagnose PHACE definitively, a patient must have a segmental IH, or an IH of more than 5 cm on the face or scalp along with one major or two minor criteria. The diagnosis is considered as “possible PHACE” if presentation includes an IH of more than 5 cm on the face or scalp and one minor criterion. Contingencies in the new criteria exist to diagnose possible PHACE in IH of the neck, chest or upper extremities, or without any discernible cutaneous IH. A neck or chest IH, in association with one major or two minor criteria, can be used to document possible PHACE. If no IH is present, two major criteria must be met (Table 1).
Structural brain and cerebrovascular anomalies are the most common and most potentially devastating extracutaneous manifestations of PHACE, and can result in significant short and long-term morbidity. Neurologic abnormalities can be divided between congenital malformations of the brain and cerebral vasculature, and progressive stenoses and occlusive disease. Structural anomalies of the brain parenchyma most commonly involve posterior fossa malformations such as Dandy-Walker complex or hypoplasia of the cerebellum. Hypoplasia or agenesis of the cerebrum, corpus callosum, septum pellucidum, and vermis are reported, as are microcephaly, heterotopia, and empty sella turcica. Multiple anomalies of the cerebral vasculature can occur, and range from hypoplasia or absence of major cerebral vessels to abnormal origin or course of such vessels. Persistent embryonic arteries, aneurysms, moya-moya-like disease, and sinus pericranii may also occur. Infants have also been reported to have progressive vascular disease resulting in ischemic stroke [5, 13]. Dysplasia of cerebral arteries is the most common anomaly, occurring in more than half of patients with abnormal cerebral vasculature. Hypoplasia or blockage and abnormal origin and course occur slightly less frequently [5, 25]. Arterial anomalies ipsilateral or bilateral to the cutaneous IH are much more common than those involving the venous system, and those vessels most frequently affected are the internal carotid artery, middle cerebral artery, anterior cerebral artery, posterior cerebral artery, and the basilar and vertebral arteries [25, 31]. These multiple abnormalities can result in seizures, developmental delay, and late-onset migraine.
The most common cardiovascular abnormality is coarctation of the aorta, and in PHACE syndrome, the coarctation is thought be unique in that right-sided aortic arch is significantly more common, and the transverse aortic arch is most often involved with associated aneurysm and abnormal brachiocephalic vessels [31]. Ventral septal defect is included as a minor criterion for diagnosis. Ophthalmologic abnormalities are less common, and it should be noted that astigmatism associated with an overlying IH does not constitute a criterion for diagnosis. Hypoplasia of the optic nerve, persistence of fetal vessels, morning glory disc anomaly, and microphthalmia are the most commonly reported eye findings. Sternal clefting and supraumbilical raphe represent ventral developmental anomalies.
Macrocephaly-capillary malformation
The syndrome, now known as macrocephaly-capillary malformation, was initially described by separate authors in 1997, Moore et al. and Clayton-Smith et al., and was initially known under the name macrocephaly-cutis marmorata telangiectatica congenita [6, 33]. Support had been building in the literature for the last several years after the proposal by Toriello and Mulliken [47] that subsequently clarified that the vascular malformation associated was neither cutis marmorata nor cutis marmorata telangiectatica congenita, and instead argued that the majority of patients have capillary malformations (CM)—commonly referred to as “port wine stains” or “salmon patches”—which are often reticular but which may be confluent, most commonly seen on the central face (confluent), limbs, and trunk. Unlike CMTC, there is no associated atrophy, ulceration, or limb hypotrophy. In addition to macrocephaly greater than the 95th percentile and the aforementioned capillary malformations, patients can have asymmetry of the brain, face, and limbs; somatic overgrowth; cognitive, speech or motor developmental delay and other neurologic abnormalities including neonatal hypotonia; limb ray defects including syndactyly or polydactyly; soft, thick, doughy skin; laxity of joints; and characteristic facial anomalies [28]. The CM of M-CM often exhibit marked fading in the first several years of life.
Similar to PHACE syndrome, both structural cerebral anomalies and functional progressive neurologic disease have been described. In M-CM, the progressive brain abnormalities are due to rapid brain growth in the first few years of life [10]. Structural brain abnormalities in M-CM include progressive ventriculomegaly (obstructive or non-obstructive), which is reported in more than half of the cases. Cerebral asymmetry, progressive white matter abnormalities, and acquired cerebellar tonsillar herniation (CTH) or Chiari type I malformations are the other neuroradiologic findings most commonly reported. Ventricular asymmetry, cavum septum pellucidum, cortical dysplasia, and polymicrogyria are also reported, with slightly lesser frequency. Polymicrogyria is most often observed in the perisylvian region. Thick optic nerve sheaths can also be seen [10, 49]. The CTH is associated with early rapid brain growth causing crowding in the posterior fossa, and therefore, is an acquired event in the majority of cases. Both ventriculomegaly and CTH are age dependent and on repeat imaging, change from the neonatal period to infancy and early childhood as a result of brain growth. Similarly, Conway et al. suggest that white matter disease is a function of abnormal and delayed myelination in early life. These authors also note in their series of 17 patients, that progressive enlargement of venous sinuses occurs during the first years of life, and is absent in the neonatal period [10, 46].Based on their findings, Conway et al. suggest magnetic resonance imaging (MRI) at diagnosis, bi-yearly for the first 2 years of life, and again at age three. Rapid increase in head circumference, seizures, new-onset paresis, focal neurologic findings, signs of increased intracranial pressure such as lethargy or headache, or oculomotor abnormalities should prompt evaluation by neurology, neurosurgery, and new neuroradiologic imaging.
Cardiac abnormalities appear to be rare, but have been reported and include arrhythmias such as atrial flutter and complex congenital heart disease. Baseline electrocardiogram is recommended to rule out arrhythmia. In a review of 112 cases, two cases of Wilms tumor were reported, which is significantly higher than that expected in the general population (1 in 10,000). Wright et al. recommend abdominal ultrasound repeated every 3–6 months until 7 years of age [49].
Gripp et al. have recently suggested that megalencephaly polymicrogyria-polydactyly hydrocephalus syndrome exhibits clinical features that overlap with M-CM, and that the two may involve a similar functional genetic pathway or gene mutation [20]. As the genetic underpinnings of both disorders remain to be elucidated, further research is necessary to prove or disprove this hypothesis.
Capillary malformation-arteriovenous malformation and RASA1 mutations
Capillary malformation, presenting as a red macular stain that can darken over years, is a common birthmark that affects about 0.3% of newborns [26]. Even though most of these lesions have a sporadic occurrence, recently, a series of heritable CMs with variable phenotypic expression caused by mutations in RASA1 have been discovered. RASA1 encodes the protein RAS p21 protein activator 1, a GTPase activating protein which negatively regulates Ras activity by converting it to an inactive state [16, 48]. Essentially, the gene products are involved in the signaling pathway of various growth factor receptors regulating cell survival, cellular proliferation, adhesion, and migration [4, 27, 51]. Mutations in RASA1 result in loss of function, therefore, there’s an increased level of activated Ras, which has been linked to various tumors. By interaction with glucocorticoid receptor DNA binding factor 1, this RasGTP has also been implicated in establishing cell polarity by regulating actin cytoskeleton in response to wounds [27]. Furthermore, a deficient vascular network is found in mutant knockout mice lacking this RAS p21 activator 1 protein [22].
Heterozygous inactivating RASA1 mutations, located on 5q13.3, were initially identified in six families exhibiting atypical, multifocal, small (1–2 cm diameter) round or ovoid cutaneous CMs in association with high-flow lesions, including arteriovenous malformation (AVM) and arteriovenous fistula (AVF) [14], which the authors dubbed capillary malformation-arteriovenous malformation. These high-flow lesions may be located in the soft tissue, bone or brain, and can hence cause life-threatening complications due to the risk of hemorrhage. In the initial investigation, a RASA1 mutation was documented in one of the families in a case of Parkes Weber syndrome, a syndrome characterized by limb hypertrophy, extensive overlying CM, and underlying AVM/AVF in the affected extremity; it was previously thought to occur sporadically, rather than as a heritable disorder [34]. More extensive study in 44 families subsequently revealed that a third of patients with multifocal CMs have associated fast-flow lesions, either AVM/AVF, including vein of Galen aneurysmal malformation, or Parkes Weber syndrome (PWS) [4, 41].
Additional novel mutations have been found in RASA1 that result in variable phenotypic expression. For example, an infant of Jewish Ashkenazi descent presenting with cutaneous CM in the left lower extremity, neck, and lower abdomen was described in association with left leg hypertrophy without evidence of AVM or AVF [24]. The symptomatology was suggestive of Parkes Weber syndrome and possibly Klippel-Trenauney syndrome, the latter characterized by cutaneous CMs associated with soft tissue and bony hypertrophy of the affected limb, varicose veins, and underlying venous and/or lymphatic malformations. Further characterization of three families comprised of 14 individuals with CMs by the same group demonstrated RASA1 mutations may also cause hereditary CMs without associated AVMs [23].
Sturge-Weber syndrome
Sturge-Weber syndrome (SWS), also called encephalotrigeminal angiomatosis, is a rare congenital neurocutaneous disorder that occurs sporadically at 1 in 40,000–50,000 live births [45]. It is characterized by angiomatous vascular malformation of the face, eye, and central nervous system, and is classified into three types according to the variable extent of involvement [42]. Type I is most common, with a classic manifestation of facial CM–commonly called port wine stain–and intracranial leptomeningeal vascular malformation, with or without ocular abnormalities such as glaucoma. Type II involves facial CM and possible glaucoma, but no brain abnormalities. Type III is characterized by leptomeningeal angiomatosis without cutaneous lesion or ocular abnormalities.
The underlying pathogenesis has been most widely postulated to reflect primary venous dysplasia, with failure of regression of primordial embryonic venous plexus that is normally present at 5–8 weeks of gestation. It has been suggested that spontaneous somatic mutation occurs during this period of embryonic development [7], although, to date, no specific genetic abnormality has been identified. That the primitive embryonic venous plexus develops around the cephalic portion of the neural tube and under the ectoderm in the region that is destined to form the facial skin offers an explanation of the association of facial CM and abnormal vascularity of the ipsilateral brain.
Facial CM is present at birth, and may be the first indication of possible SWS. The port wine stains are generally flat, pink and blanch under pressure, and may gradually darken in color in later childhood or adulthood. They are made of ectatic dermal capillaries and small venules, and found to have diminished perivascular innervations. However, only about 8% of individuals with port wine stains have intracranial involvement [44]. If the CM involves the forehead to one side or the eyelid, the risk of brain and eye involvement becomes higher, ranging 10–35%. In SWS, the CM is most common in the trigeminal I (V1) sensory distribution, but may be more widespread extending to the second (V2) and third (V3) divisions of trigeminal distribution of the face, and occasionally involving the neck and trunk (Fig. 2). Bilateral involvement occurs in up to 20% of cases [39]. No association has been found between the presence or extent of facial CM and neurological symptoms [11, 38]. Five to fifteen percent of individuals affected by SWS, in fact, have leptomeningeal vascular malformations without cutaneous stigmata (type III) [7, 37].
In patients with SWS involving the central nervous system, seizures typically develop within the first year of life. While the greatest neurologic decline commonly occurs during the first few years of life, neurologic deterioration with stroke-like episodes can occur into adulthood [29]. The fundamental central nervous system abnormality in SWS is venous stasis due to the lack of normal cortical venous development, and a persistent but ineffectual embryonic venous plexus that leads to a hypoperfused state in the affected brain parenchyma, with progressive inability to meet the metabolic demands, particularly in the presence of seizures [29]. Patients with early onset of epilepsy are more likely to have hemiparesis, status epilepticus, and developmental delay compared to those whose seizures start later in life [3]. Other neurological symptoms and signs include mental retardation, headaches, and stroke-like symptoms presenting with hemiparesis and visual field defects [11, 38]. Glaucoma is the most common ocular complication of SWS, affecting 30–70% of patients with a bimodal presentation: 60% develop in infancy and 40% in childhood to early adulthood [43].
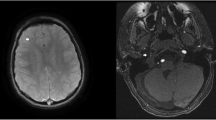
Neuroimaging is important in establishing intracranial involvement. Both computed tomography (CT) and MRI have been used to provide morphologic information. CT is particularly useful for depicting dystrophic calcification in the involved brain region, typically in a tram-track appearance along the cortex reflecting cortical laminar calcification (Fig. 3a), but may also be present as a finer calcification that is predominantly distributed in the subcortical white matter (Fig. 3b). In addition to calcification, brain atrophy can be readily identified on CT. The regions of brain involvement are most commonly the parietal and occipital lobes, gradually progressing into the frontal and temporal lobes resulting in hemiatrophy. Gadolinium-enhanced MRI is considered the most sensitive modality for depicting leptomeningeal enhancement, reflective of the pial angiomatosis that is the hallmark of SWS (Fig. 4) [17, 29]. Increased permeability of the dysplastic vessels in SWS suggested by several authors [11, 12] may explain the superior depiction of pial angiomatosis on post-contrast FLAIR images [19]. Contrast-enhanced MRI is also useful for revealing sometimes subtle bilateral hemispheric involvement (Fig. 5), compensatory venous drainage pathways via transmedullary venous malformations, enlarged choroid plexus glomus, as well as cerebellar and eye involvement. However, diagnosis in presymptomatic infants remains challenging, as initial CT and MRI in the newborn period are usually negative [8, 50].
Different calcification patterns in SWS demonstrated on unenhanced head CT. a Thick tram-track calcification along the right occipital–parietal cortex in a 20-year-old male with SWS, and recurring episodes of headache and speech arrest. b Fine calcification in the subcortical white matter underlying atrophic left frontal lobe in a 3-month-old male infant who was diagnosed with SWS at birth. He had a port wine stain overlying the left side of his face down to the upper lip and presented with increasing seizures
MRI of the same patient in Fig. 3a. a Axial T2-weighted image shows mild focal atrophy in the right occipital lobe, associated with dark signal along the cortex reflecting cortical calcification. b Post-Gd T1-weighted image shows leptomeningeal enhancement in the same region, and an enhancing enlarged choroid plexus glomus in the right atrium
Functional neuroimaging with single photon emission computed tomography (SPECT) and positron emission tomography (PET) is useful for depicting deficits in cerebral perfusion and metabolism. Cerebral perfusion imaging using 99mtechnetium hexamethylpropyleneamineoxime SPECT demonstrated hypoperfusion in the area of vascular malformation, at times more extended compared to abnormalities evident on CT and MRI [2, 18]. In a few cases, the perfusion (estimated using SPECT) and metabolism (assessed using [18F] fluorodeoxyglucose PET) abnormalities were evident before the development of structural abnormalities [40], suggesting that functional measures may be more sensitive for early diagnosis of cerebral involvement. Furthermore, longitudinal studies were useful in identifying regional reductions in perfusion and glucose metabolism in young children within the first 2 years of life that corresponded to the patient’s patterns of neurological deterioration [30]. Impaired autoregulation of blood flow to meet metabolic demand during seizures in SWS patients is suggested by the absolute reduction in blood flow in the involved or remote brain regions on ictal SPECT studies [1, 35].
Treatment is primarily targeted to symptoms, particularly in controlling seizures. That seizures may exacerbate brain injury in SWS strongly argue for more aggressive epilepsy management, particularly early in the course of the disease [3, 9]. In addition to anticonvulsants, current practice also advocates administration of low-dose aspirin at the time of diagnosis, upon or prior to the onset of first seizure or other focal neurological deficits, with the rationale that this will prevent the progression of impaired cerebral flow and further brain injury [8, 9]. Finally, surgical lobectomy or hemispherectomy may also be indicated in cases where seizures are refractory to medical therapy. A retrospective surgical series of 27 children suggests that early surgery is important in improved developmental outcomes [3]. Seizure control was best correlated with the degree of resection of diseased brain tissue, but not patient age at the time of surgery.
References
Aylett SE, Neville BG, Cross JH, Boyd S, Chong WK, Kirkham FJ (1999) Sturge-Weber syndrome: cerebral haemodynamics during seizure activity. Dev Med Child Neurol 41:480–485
Bar-Sever Z, Connolly LP, Barnes PD, Treves ST (1996) Technetium-99m-HMPAO SPECT in Sturge-Weber syndrome. J Nucl Med 37:81–83
Bourgeois M, Crimmins DW, de Oliveira RS, Arzimanoglou A, Garnett M, Roujeau T, Di Rocco F, Sainte-Rose C (2007) Surgical treatment of epilepsy in Sturge-Weber syndrome in children. J Neurosurg 106:20–28
Brouillard P, Vikkula M (2007) Genetic causes of vascular malformations. Hum Mol Genet 16 Spec No 2:R140–R149
Burrows PE, Robertson RL, Mulliken JB, Beardsley DS, Chaloupka JC, Ezekowitz RA, Scott RM (1998) Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: report of eight patients. Radiology 207:601–607
Clayton-Smith J, Kerr B, Brunner H, Tranebjaerg L, Magee A, Hennekam RC, Mueller RF, Brueton L, Super M, Steen-Johnsen J, Donnai D (1997) Macrocephaly with cutis marmorata, haemangioma and syndactyly–a distinctive overgrowth syndrome. Clin Dysmorphol 6:291–302
Comi AM (2003) Topical review: pathophysiology of Sturge-Weber syndrome. J Child Neurol 18:509–516
Comi AM (2007) Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol 5:257–264
Comi AM (2007) Sturge-Weber syndrome and epilepsy: an argument for aggressive seizure management in these patients. Expert Rev Neurother 7:951–956
Conway RL, Pressman BD, Dobyns WB, Danielpour M, Lee J, Sanchez-Lara PA, Butler MG, Zackai E, Campbell L, Saitta SC, Clericuzio CL, Milunsky JM, Hoyme HE, Shieh J, Moeschler JB, Crandall B, Lauzon JL, Viskochil DH, Harding B, Graham JM Jr (2007) Neuroimaging findings in macrocephaly-capillary malformation: a longitudinal study of 17 patients. Am J Med Genet A 143A:2981–3008
Di Rocco C, Tamburrini G (2006) Sturge-Weber syndrome. Childs Nerv Syst 22:909–921
Di Trapani G, Di Rocco C, Abbamondi AL, Caldarelli M, Pocchiari M (1982) Light microscopy and ultrastructural studies of Sturge-Weber disease. Childs Brain 9:23–36
Drolet BA, Dohil M, Golomb MR, Wells R, Murowski L, Tamburro J, Sty J, Friedlander SF (2006) Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics 117:959–964
Eerola I, Boon LM, Mulliken JB, Burrows PE, Dompmartin A, Watanabe S, Vanwijck R, Vikkula M (2003) Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet 73:1240–1249
Frieden IJ, Reese V, Cohen D (1996) PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 132:307–311
Grewal T, Evans R, Rentero C, Tebar F, Cubells L, de Diego I, Kirchhoff MF, Hughes WE, Heeren J, Rye KA, Rinninger F, Daly RJ, Pol A, Enrich C (2005) Annexin A6 stimulates the membrane recruitment of p120GAP to modulate Ras and Raf-1 activity. Oncogene 24:5809–5820
Griffiths PD (1996) Sturge-Weber syndrome revisited: the role of neuroradiology. Neuropediatrics 27:284–294
Griffiths PD, Boodram MB, Blaser S, Armstrong D, Gilday DL, Harwood-Nash D (1997) 99 mTechnetium HMPAO imaging in children with the Sturge-Weber syndrome: a study of nine cases with CT and MRI correlation. Neuroradiology 39:219–224
Griffiths PD, Coley SC, Romanowski CA, Hodgson T, Wilkinson ID (2003) Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol 24:719–723
Gripp KW, Hopkins E, Vinkler C, Lev D, Malinger G, Lerman-Sagie T, Dobyns WB (2009) Significant overlap and possible identity of macrocephaly capillary malformation and megalencephaly polymicrogyria-polydactyly hydrocephalus syndromes. Am J Med Genet A 149A:868–876
Haggstrom A, Lammer E, Schneider R, Marcucio R, Frieden I (2006) Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics 117:698–703
Henkemeyer M, Rossi DJ, Holmyard DP, Puri MC, Mbamalu G, Harpal K, Shih TS, Jacks T, Pawson T (1995) Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature 377:695–701
Hershkovitz D, Bercovich D, Sprecher E, Lapidot M (2008) RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol 158:1035–1040
Hershkovitz D, Bergman R, Sprecher E (2008) A novel mutation in RASA1 causes capillary malformation and limb enlargement. Arch Dermatol Res 300:385–388
Heyer GL, Dowling MM, Licht DJ, Tay SK-H, Morel K, Garzon MC, Meyers P (2008) The cerebral vasculopathy of PHACES syndrome. Stroke 39:308–316
Jacobs A, Walton R (1976) The incidence of birthmarks in the neonate. Pediatrics 58:218–222
Kulkarni SV, Gish G, van der Geer P, Henkemeyer M, Pawson T (2000) Role of p120 Ras-GAP in directed cell movement. J Cell Biol 149:457–470
Lapunzina P, Gairi A, Delicado A, Mori MA, Torres ML, Goma A, Navia M, Pajares IL (2004) Macrocephaly-cutis marmorata telangiectatica congenita: report of six new patients and a review. Am J Med Genet A 130A:45–51
Maria B, Hoang K, Robertson R, Barnes P, Drane W, Chugani H (1999) Imaging brain structure and function in Sturge-Weber syndrome. In: Bodensteiner J, Roach E (eds) Sturge-Weber syndrome. Sturge-Weber Foundation, Mt Freedom, pp 43–69
Maria BL, Neufeld JA, Rosainz LC, Drane WE, Quisling RG, Ben-David K, Hamed LM (1998) Central nervous system structure and function in Sturge-Weber syndrome: evidence of neurologic and radiologic progression. J Child Neurol 13:606–618
Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J, Frieden I, Drolet B (2009) Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 124:1447–1456
Metry DW, Haggstrom AN, Drolet BA, Baselga E, Chamlin S, Garzon M, Horii K, Lucky A, Mancini AJ, Newell B, Nopper A, Heyer G, Frieden IJ (2006) A prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complications. Am J Med Genet A 140A:975–986
Moore C, Toriello H, Abuelo D, Bull M, Curry C, Hall B, Higgins J, Stevens C, Twersky S, Weksberg R, Dobyns W (1997) Macrocephaly-cutis marmorata telangiectatica congenita: a distinct disorder with developmental delay and connective tissue abnormalities. Am J Med Genet A 70:67–73
Mulliken J, Young A (1988) Vascular birthmarks: hemangiomas and malformations. Saunders, Philadelphia
Namer IJ, Battaglia F, Hirsch E, Constantinesco A, Marescaux C (2005) Subtraction ictal SPECT co-registered to MRI (SISCOM) in Sturge-Weber syndrome. Clin Nucl Med 30:39–40
Pascual-Castroviejo I (1978) Vascular and nonvascular intracranial malformation associated with external capillary hemangiomas. Neuroradiology 16:82–84
Pascual-Castroviejo I, Pascual-Pascual SI, Viano J, Martinez V, Coya J (1995) Sturge-Weber syndrome without facial nevus. Neuropediatrics 26:220–222
Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, Viano J (2008) Sturge-Weber syndrome: study of 55 patients. Can J Neurol Sci 35:301–307
Peterman AF, Hayles AB, Dockerty MB, Love JG (1958) Encephalotrigeminal angiomatosis (Sturge-Weber disease); clinical study of thirty-five cases. J Am Med Assoc 167:2169–2176
Reid DE, Maria BL, Drane WE, Quisling RG, Hoang KB (1997) Central nervous system perfusion and metabolism abnormalities in Sturge-Weber syndrome. J Child Neurol 12:218–222
Revencu N, Boon L, Mulliken J, Enjolras O, Cordisco M, Burrows P, Clapuyt P, Hammer F, Dubois J, Baselga E, Brancati F, Carder R, Quintal J, Dallapiccola B, Fischer G, Frieden I, Garzon M, Harper J, Johnson-Patel J, Labrèze C, Martorell L, Paltiel H, Pohl A, Prendiville J, Quere I, Siegel D, Valente E, Van Hagen A, Van Hest L, Vaux K, Vicente A, Weibel L, Chitayat D, Vikkula M (2008) Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat 29:959–965
Roach E, Bodensteiner J (1999) Neurologic manifestations of Sturge-Weber syndrome. In: Bodensteiner J, Roach E (eds) Sturge-Weber syndrome. Sturge-Weber Foundation, Mt Freedom, pp 27–38
Sujansky E, Conradi S (1995) Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol 10:49–58
Tallman B, Tan OT, Morelli JG, Piepenbrink J, Stafford TJ, Trainor S, Weston WL (1991) Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications. Pediatrics 87:323
Thomas-Sohl KA, Vaslow DF, Maria BL (2004) Sturge-Weber syndrome: a review. Pediatr Neurol 30:303–310
Thong MK, Thompson E, Keenan R, Simmer K, Harbord M, Davidson G, Haan E (1999) A child with hemimegalencephaly, hemihypertrophy, macrocephaly, cutaneous vascular malformation, psychomotor retardation and intestinal lymphangiectasia–a diagnostic dilemma. Clin Dysmorphol 8:283–286
Toriello H, Mulliken J (2007) Accurately renaming macrocephaly-cutis marmorata telangiectatica congenita (M-CMTC) as macrocephaly-capillary malformation (M-CM). Am J Med Genet A 143A:3009
Trahey M, Wong G, Halenbeck R, Rubinfeld B, Martin GA, Ladner M, Long CM, Crosier WJ, Watt K, Koths K et al (1988) Molecular cloning of two types of GAP complementary DNA from human placenta. Science 242:1697–1700
Wright DR, Frieden IJ, Orlow SJ, Shin HT, Chamlin S, Schaffer JV, Paller AS (2009) The misnomer “macrocephaly-cutis marmorata telangiectatica congenita syndrome”: report of 12 new cases and support for revising the name to macrocephaly-capillary malformations. Arch Dermatol 145:287–293
Yeakley JW, Woodside M, Fenstermacher MJ (1992) Bilateral neonatal Sturge-Weber-Dimitri disease: CT and MR findings. AJNR Am J Neuroradiol 13:1179–1182
Yue Y, Lypowy J, Hedhli N, Abdellatif M (2004) Ras GTPase-activating protein binds to Akt and is required for its activation. J Biol Chem 279:12883–12889
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puttgen, K.B., Lin, D.D.M. Neurocutaneous vascular syndromes. Childs Nerv Syst 26, 1407–1415 (2010). https://doi.org/10.1007/s00381-010-1201-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1201-3