Abstract
Objectives
Thalamic tumors are uncommon, and although gross total removal (GTR) is a prospective goal, its interest is debated because the thalamus constitutes a highly functional region. The relation of choice of the surgical approach, achievability of GTR, and operative morbidity to the anatomic location of the tumor has received little attention in the medical literature.
Materials and methods
We reviewed retrospectively the cases of pediatric patients treated for thalamic tumor, with pre- and postoperative magnetic resonance imaging, and who were operated with the aim of maximal surgical removal.
Conclusion
We reviewed 16 cases operated between 1992 and 2003. The clinical presentation was dominated by intracranial hypertension and hemiparesis. Fifteen children were operated through transcortical approaches: transfrontal in six cases, transparietal in six, and transtemporal in three. The remaining patient was operated through an infratemporal approach. All operations performed since 1998 used intraoperative neuronavigation. Complete or near-total resection was achieved in 11 cases; only subtotal resection was achieved in the remaining five cases. The most common postoperative morbidity was visual field defect. Hemiparesis was unchanged or improved in all the cases. Seven children died of tumor progression, in relation with high histological grade, and one died of acute hydrocephalus. The approach to thalamic tumors needs to be planned according to the location of critical neural structures. GTR of thalamic tumors in children bears acceptable morbidity and may even improve preoperative deficits. Surgery alone can be curative in low-grade tumors; in high-grade or infiltrating tumors, GTR is only part of the overall oncological management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thalamic tumors are rare and represent about 4% of all brain tumors [6]. Historically, thalamic tumors were considered inoperable because of the vital structures surrounding the thalamus, like internal capsule and subthalamus, and the risk of major postoperative morbidity. However, since the emergence of MRI and the availability of image guided surgery, thalamic tumors have become more likely targets for surgical resection. Data from the literature suggest that a more aggressive surgical resection is associated with improved survival [1]. Pediatric neurosurgeons have shown more aggressiveness in approaching thalamic tumors because thalamic tumors are relatively more common in children, and children have a higher degree of neuroplasticity, allowing a better recovery than adults. Thalamic tumors can be resected through several approaches; however, the postoperative morbidity and rate of resection obtained through the different approaches are poorly documented because the number of cases published is limited [1, 13, 20].
To discuss the indications for surgery and the choice of surgical approach for thalamic tumors, we reviewed our experience with resective surgery in thalamic tumors in children.
Materials and methods
Our institution is the only referral center for pediatric neurosurgery in a 4-million population area with a yearly accrual of 80 new pediatric tumor cases. We selected from our database the cases of children treated for a unilateral thalamic tumor, diagnosed with MRI, and operated with the goal of maximal tumor resection.
We reviewed independently the preoperative and postoperative magnetic resonance imaging (MRI) films to determine the epicenter of the tumor, its extension to neighboring structures, and the extent of tumor removal. We did not include cases with a tumor involving the thalamus but not originating from it, nor did we include bilateral tumors. The extent of tumor resection was evaluated both with postoperative MRI and the surgeon’s observation noted on the operating report. We defined gross total resection as absence of macroscopic rest after surgery; near-total resection as less than 1.5 cm3 residual tumor volume; and subtotal resection as anything less than that.
The patients were all followed in outpatient clinics, both in neurosurgery and in the oncology department, and underwent formal neuropsychological, neuroophthalmological, and neuroendocrinological evaluation, as required according to the location of the tumor, surgical approach, and the use of chemotherapy and/or irradiation.
Results
Clinical presentation
Between 1992 and 2003, we operated 16 children for thalamic tumors. The mean age at the time of tumor resection was 7.4 ± 4.4 years (2.4–14.9); the male/female ratio was 1:1.28. The most common presenting symptom was increased intracranial pressure, found in 12 cases, in relation with the tumor mass in eight cases, and with hydrocephalus in four cases (Fig. 1). Other signs and symptoms were hemiparesis in nine cases (Fig. 2), visual dysfunction in seven cases, with abducens nerve palsy for one child, and visual field defect for one other child. Three patients had movement disorders (tremor or dystonia) related to disturbance of the extra pyramidal pathway (Fig. 3). Four patients presented acutely because of intra-tumoral bleeding, all of them needed operation in emergency because of acute increased intracranial pressure (three cases) or rapid worsening of hemiparesis (one case). The signs and symptoms at presentation are summarized in the Table 1. In all the cases in which preoperative MRI was in favor of high grade tumor, the surgical indication was decided because of a severe intracranial hypertension, as a base to other oncological therapeutic, because of non-response to chemotherapy, or with the hope to reduce the tumoral volume for a better response to adjuvant treatment. The objective for the low-grade tumor was to be curative with surgery alone.
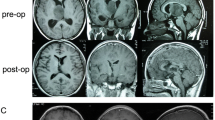
Case 15: 12-month female presenting with severe intracranial hypertension associated with a right hemiparesis and dystonia, revealing a left thalamic tumor with obstructive hydrocephalus, which initially extended into the third ventricle only (a axial T1-weighted MRI; b coronal T2-weighted MRI). Ventriculocisternostomy was performed in emergency, during which the tumor could be biopsied. The histopathological diagnosis was oligoastrocytoma grade II. Initial treatment was chemotherapy, which obtained initially a stabilization of the tumor. Two years later, however, the tumor had progressed and caused new motor deficit (c and d axial and coronal contrast-enhanced T1-weighted MRI). Because the tumor had become accessible from its superior aspect, tumor resection was performed using a transfrontal transventricular approach, allowing resection of roughly two third of the tumor volume (e and f axial and coronal contrast-enhanced T1-weighted MRI) with partial recovery of the deficit. Then, after postoperative irradiation and new chemotherapy, a partial response was obtained and the patient is clinically stable
Case 10: 3-year female presenting with acute left hemiparesis in relation with a right thalamic tumor with intra-tumoral bleeding. Tumor resection through a transcortical transtemporal approach, guided by the neuronavigation, was total. Postoperatively, the patient improved partially and has marked dystonia in her left upper limb. Histopathological diagnosis was pilocytic astrocytoma. After 1 year, chemotherapy was administered because of tumor progression, which achieved stabilization of the tumor (a preoperative CT scan; b and c preoperative axial and coronal contrast-enhanced T1-weighted MRI; d and e postoperative axial and coronal contrast-enhanced T1-weighted MRI)
Case 7: 4-year left-handed male presenting with intentional tremor and dystonia of the right hand. He was operated though a transcortical transparietal approach guided by neuronavigation, which allowed gross-total removal. Postoperatively, the patient was unchanged. Histopathological diagnosis was high-grade oligodendroglioma. In spite of chemotherapy then irradiation, the tumor progressed with leptomeningeal spread leading to death 4 months after surgery. a and b Preoperative axial and sagittal contrast-enhanced T1-weighted MRI. c and d Postoperative axial and sagittal contrast-enhanced T1-weighted MRI
MRI anatomical study
The epicenter of the tumor was located in the anterior thalamic nuclei in five cases, in the lateral nuclei in three cases, in the medial nuclei in two cases, and in the pulvinar in six cases. Tumor extended toward the globus pallidus and the hypothalamus in three cases, the crus cerebri in five cases, and toward the atrium of the lateral ventricle and the parietal and temporal lobes in three other cases (all of them emerging from the pulvinar).
T2-weighted images were the most accurate to study the relation of the tumor to the internal capsule and basal ganglia. If these structures appeared infiltrated by the tumor, we elected stereotactic biopsies first, followed by radiotherapy and/or chemotherapy. In the other cases, surgical removal of the tumor was decided, especially if neuroimaging was in favor of low-grade tumor. The point of origin of the tumor, its relation to the salient neural structures, and its point of emergence in the ventricles were the critical criteria for deciding surgical resection and which approach to favor.
Perioperative management of CSF problems
Significant hydrocephalus was present upon presentation in four patients. Two of these required an endoscopic ventriculo-cisternostomy in emergency, and one had a ventriculo-peritoneal shunt. The last patient had minimal univentricular dilatation with mild clinical symptoms so hydrocephalus was not treated for itself. Several other patients had some degree of moderate ventriculomegaly, which was not treated, to take advantage of this additional room to facilitate tumor resection through the transventricular approach.
After tumor resection, four other patients developed significant hydrocephalus; three of these were treated with ventriculo-peritoneal shunt; the last patient underwent endoscopic ventriculo-cisternostomy. One patient developed a subdural collection, which required subduroperitoneal shunting, and another one presented with a subcutaneous collection which required repeated subtractive punctures.
Preoperative biopsies
Two patients had biopsies before surgical removal, one in stereotaxic condition, another one (mentioned above) during endoscopic ventriculo-cisternostomy.
Tumor resection
We performed a transcortical transventricular approach in 15 cases: through the frontal lobe in six cases, through the parietal lobe in six, and through the temporal lobe in three; in the last case, the tumor was approached through an infratemporal supratentorial approach. Neuronavigation was used in eight cases. Since the neuronavigation became available, it has been used in all new cases, except for those operated in emergency. The extent of tumor resection was gross-total in nine cases, near-total in two, and subtotal in five.
Perioperative morbidity and mortality
No patient died during the perioperative period. Four children had a visual field defect after surgery, but only one of these had a preoperative instrumental exploration of his visual field showing preserved visual field. The child operated by the infratemporal approach had a trochlear nerve palsy after surgery, which eventually required surgical correction. Four patients had movement disorder postoperatively: two had tremor, one had ataxia, and one had dystonia; however, in some of these, severe intracranial hypertension or heavy hemiparesis before surgery precluded complete neurological examination, including the search for movement disorder. In two patients, severe spasticity required treatment by botulinum toxin; both presented with massive hemiparesis before surgery.
When present before operation, hemiparesis was improved postoperatively in all cases. One patient presented with transitory worsening of his hemiparesis, which was spontaneously regressive in 2 weeks; there was no new deficit. No patient had seizures requiring antiepileptic medication.
Histopathological findings, adjuvant treatment and survival
Four patients had a low-grade glioma (pilocytic astrocytoma), seven had high grade glioma, two had an ependymoma, two had a primitive neuroepithelial tumor, and one had a ganglioneurocytoma. With the exception of pilocytic astrocytomas, adjuvant therapy was administered in the majority of patients and included radiotherapy in four cases, radiotherapy plus chemotherapy in six cases, and chemotherapy alone in four cases. The mean follow-up was 35.9 months (2 to 128). Eight patients died: seven from tumor progression (all associated with high histopathological grade) and the last one because of obstruction of his ventriculo-peritoneal shunt. These data are summarized in Table 2. There is no statistical correlation between survival and location of the tumor, adjuvant treatment, or using neuronavigation probably because of the small number of patients with this uncommon pathology.
Discussion
Evolution of treatment towards maximal surgical removal
There are few data in the literature on the clinical outcome of patients undergoing resective surgery for thalamic tumors after resective surgery because these tumors are uncommon and are rarely operated on. For that reason, all series are retrospective, with a variety of different histopathological diagnoses [1, 4, 6, 13, 20]. In addition, tumor location is often unclear in the older published cases because of the lack of high-quality MRI. The subject of our study was tumors originating from the thalamus in children and the evaluation of resective surgery.
The first reported pediatric series involving thalamic tumors was published by Bernstein et al. [4] in 1984. Patients underwent stereotactic or open biopsy or partial resection, and no total resection was achieved. In 1987, Beks et al. [3] recommended stereotactic biopsy followed by irradiation in selected cases and proposed to perform partial resection, only if necessary, to decrease intracranial pressure (ICP). In 1994, Villarejo et al. [17] recommended surgical treatment and preferred “craniotomy to stereotactic biopsy because with that technique it is possible to achieve total or subtotal removal”. In the year 2000, Steiger et al. [15] stated that “thalamic gliomas can be surgically removed with an acceptable risk”. Regarding the relevance of indications to histopathology, Cuccia and Monges estimated “that both low-grade and anaplastic thalamic tumors must be operated on; benign astrocytoma can be cured, and the survival rate of patients with anaplastic tumor could be increased” [1]. On the contrary, other teams consider that the appropriate surgical management of high-grade thalamic glioma should be limited to the control of raised ICP and a stereotactic or endoscopic biopsy [2]. From this short literature review, gross-total resection of thalamic tumors appears to gain momentum, at least for pediatric cases. The oncological outcome appears generally to benefit from GTR, and the emergence of computer-assisted surgery has made the approach to these tumors safer, allowing a more aggressive attitude.
Clinical presentation
Clinical presentation of our patients was in keeping with data from the literature. In the series from Albright [1], the most common sign associated with such tumors was contralateral paresis. Despite the role of the thalamus in movement regulation, thalamic tumors were uncommonly associated with movement disorders in our series. For the majority of authors, raised ICP and motor deficit are the most common symptoms [6, 8, 10–12, 17, 18]. Raised ICP is due to the volume of the neoplasm or to obstructive hydrocephalus related to intraventricular growth and compression of midline structures. In our experience, the motor deficit was often improved after tumor resection, suggesting that it was due to compression rather than invasion of the internal capsule. Thalamic pain, dystonia, or tremor are unusual on presentation and are also uncommon complications of surgery.
Radiological examination and anatomical evaluation
Since its introduction 20 years ago, MRI has greatly enhanced the possibility of surgical resection of thalamic tumors, demonstrating their precise location and accessibility and allowing precise evaluation of the resection on postoperative studies. Mass effect due to the tumor and related edema usually results in the displacement of adjacent cerebrospinal fluid (CSF) spaces and stretching of the internal capsule [5]. In the near future, evaluation with diffusion tensor tractography will certainly be a great adjunct to the preoperative evaluation of these cases.
Management of hydrocephalus
CSF circulation is a problem before and after tumor resection. Despite the poor results after preoperative shunts in thalamic tumors in the series presented by Goel [7] who reported neurological degradation after shunting in 8/31 patients, in our series, no patient was worsened after shunt insertion. However, the fact that one of our patients died after shunt obstruction confirms the particular severity of obstructive hydrocephalus due to thalamic tumors. The ventricular enlargement can also be viewed as an advantage because it helps gain access to the tumor, and, if well tolerated, should often be respected until tumor resection.
Surgical approach and morbidity
The thalamus is a large mass of gray matter which is limited ventrally by the hypothalamic sulcus, which separates it from the hypothalamus anteriorly and from the subthalamus posteriorly. The lateral aspect of the thalamus is in contact with the internal capsule and thalamic radiations. The caudal part of the thalamus overlies the midbrain structures. As a result, from a surgical standpoint, only the ventro-lateral aspect of the thalamus represents a forbidden zone inaccessible to surgery [13, 15]. The posterior aspects of the thalamus (facing the posterior commissure and the aqueduct), its medial aspect (related to the third ventricle) and its superior aspect (bounded by the stria medullaris, related to the lateral ventricle and the tela choroidea), are free surfaces. These surfaces are enlarged and made more accessible by the development of the tumor. As the ventricles are often enlarged on account of obstructive hydrocephalus, the choice of transventricular approaches for the resection of tumors of the thalamus often appears to be self-evident.
Several surgical nuances can be proposed. For tumors in the superior thalamus compressing the frontal horn and elevating the ependyma of the body of the lateral ventricle, Vajda [16] proposed a precentral–transcallosal approach. We prefer transcortical frontal approaches because access through the transcallosal approach can be limited laterally by stretching of the pericallosal artery. Prakash [14] also preferred to make a small paramedial cortical incision in the parietal lobe, gain access medially to the interhemispheric fissure, and continue as a classical transcallosal approach to spare the functionally important cortical veins. For tumors involving the pulvinar, a posterior interhemispheric–parasplenial–transventricular approach has been proposed by Yasargil [20], which is a variation on the posterior–interhemispheric–transcallosal or transtentorial approach used for pineal region tumors [9]. This latest approach is particularly interesting if the tumor of the pulvinar extends posteriorly or inferiorly, and could possibly be combined with a parietal–transcortical approach. The infra-tentorial-supracerebellar approach is hailed as less invasive than others because it is completely extra-axial; however, the window between the two basal veins of Rosenthal is limited, and this approach is not adequate for tumors extending more than one centimeter laterally [15]. Yasargil [19] also proposed a pterional transsylvian transinsular approach for tumors within the ventral posterior thalamic region, especially when there is a close relationship between the insula and the thalamic tumor. A short corticotomy is made through the mid-portion of the post-central sulcus of the insula, and the tumor can then be identified beneath the insular cortex. The choice of the approach thus depends not only on the location of the tumor but also to a large extent on the experience of the neurosurgeon.
Like other clinical series [4], there was no operative death in our series, nor did we record permanent worsening of the patient’s neurological status. These surprisingly favorable clinical results may be ascribed to the exceptional neuroplasticity of the pediatric population and may not be reproducible in adults. The most common postoperative morbidity was partial hemianopsia, like in other series in which parieto-occipital transventricular approach was used [15]. Despite using transcortical routes in 16/17 cases and not using prophylactic antiepileptic treatments, we observed only one case of postoperative seizure.
Role of tumor resection in the multidisciplinary approach of thalamic tumors
Our study indicates that resective surgery in the thalamus is possible with acceptable morbidity in children and even with clinical improvement in many cases. As many of these patients present with raised intracranial pressure on admission, surgical decompression may be the safest way to initiate the oncological treatment of these tumors. In low-grade gliomas, gross-total or near-total resection may be the sole treatment; in high-grade tumors, tumor resection allows relief of the intracranial hypertension, but we must be aware that surgery cannot cure the patient unless the chemo- and radiotherapy can prevent recurrence.
In some cases, the tumor is initially inaccessible, and the initial treatment is chemotherapy and/or irradiation based on the diagnosis obtained by biopsy. Secondarily, however, if the tumor does not respond to treatment, its enlarged volume can make it accessible to surgical resection, like in the case illustrated in the Fig. 1, who was operated secondarily, with subtotal resection followed by irradiation and a favorable outcome.
Our philosophy is that surgical resection of thalamic tumors is, along with chemotherapy, irradiation, and techniques of CSF diversion, part of an armamentarium which has to be adapted according to the needs of the long-term control of these difficult tumors. This stresses the need for a close collaboration between the neurosurgical and oncological teams.
Conclusion
For tumors in this rare location, resective surgery is possible and often desirable. Transventricular approaches are often preferred, the choice of the cortical incision depending on the epicenter of the tumor, its maximal development, and the size of the ventricular system. Surgical resection is an important element of the overall oncological management of these difficult tumors.
References
Albright AL (2004) Feasibility and advisability of resections of thalamic tumors in pediatric patients. J Neurosurg (Pediatrics 5) 100:468–472
Allen JC (2000) Initial management of children with hypothalamic and thalamic tumors and the modifying role of neurofibromatosis-1. Pediatr Neurosurg 32:154–162
Beks JWF, Bouma GJ, Journée HL (1987) Tumours of the thalamic region. Acta Neurochir 85:125–127
Bernstein M, Hoffman HJ, Halliday WC, Bruce Hendrick E, Humphreys RP (1984) Thalamic tumors in children. J Neurosurg 61:656–659
Colosimo C, di Lella GM, Tartaglione T, Riccardi R (2002) Neuroimaging of thalamic tumors in children. Childs Nerv Syst 18:426–439
Cuccia V, Monges J (1997) Thalamic tumors in children. Childs Nerv Syst 13:514–521
Goel A (2000) Preoperative shunts in thalamic tumours. Neurol India 48:347–350
Hirose G, Lombroso CT, Eisenberg H (1975) Thalamic tumors in childhood. Arch Neurol 32:740–744
Ishii R, Suzuki Y, Watanabe A, Mouri Y, Ishii N, Yoshii I (2002) Gross total removal of gliomas in the pulvinar and correlative microsurgical anatomy. Neurol Med Chir (Tokyo) 42:536–546
Martinez-Lage JF, Perez-Espejo MA, Esteban JA, Poza M (2002) Thalamic tumors: clinical presentation. Childs Nerv Syst 18:405–411
Mayer M, Ponsot G, Kalifa C, Lemerle J, Arthuis M (1982) Tumeurs des noyaux gris chez l’enfant. Arch Fr Pediatr 39:91–95
Nishio S, Morioka T, Suzuki S, Takeshita I, Fukui M (1997) Thalamic gliomas: a clinicopathologic analysis of 20 cases with reference to patient age. Acta Neurochir 139:336–342
Özek MM, Türe U (2002) Surgical approach to thalamic tumors. Childs Nerv Syst 18:450–456
Prakash B (1985) Surgical approach to large thalamic gliomas. Acta Neurochir 74:100–104
Steiger HJ, Götz C, Schmid-Elsaesser R, Stummer W (2000) Surgical anatomy and results of a pilot series using maximum microsurgical removal. Acta Neurochir 142:1327–1337
Vajda J (1998) Thalamic tumors in children. Childs Nerv Syst 14:349
Villarejo F, Amaya C, Perez Diaz C, Pascual A, Alvarez Sastre C, Goyenechea F (1994) Radical surgery of thalamic tumors in children. Childs Nerv Syst 10:111–114
Wald SL, Fogelson H, McLaurin RL (1982) Cystic thalamic gliomas. Child’s Brain 9:381–393
Yasargil MG (1988) Microneurosurgery, vol 3B. Thieme, Stuttgart
Yasargil MG (1996) Microneurosurgery, vol 4B. Thieme, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baroncini, M., Vinchon, M., Minéo, JF. et al. Surgical resection of thalamic tumors in children: approaches and clinical results. Childs Nerv Syst 23, 753–760 (2007). https://doi.org/10.1007/s00381-007-0299-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-007-0299-4