Abstract
Objective
To describe the different imaging modalities used for the diagnosis and classification of hydrocephalus, their role in defining the optimal treatment of hydrocephalus and to define the optimal preoperative diagnostics for endoscopic third ventriculocisternostomy (ETV).
Methods
An overview on available imaging modalities for hydrocephalus will be given and their pros and cons discussed. In addition, different aspects of the treatment of hydrocephalus by shunts and by ETV will be highlighted.
Discussion
The role of the technical aspects of performing an ETV, the role of the surgeon’s philosophy, the role of the urgency of the procedure, and the role of informed consent on the requirements for the imaging of the hydrocephalus will be discussed.
Conclusion
The authors conclude that MRI is a conditio sine qua non for ETV in elective surgical cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of different classification schemes for various types of hydrocephalus were proposed over the years [55]. These schemes were based on anatomy, radiological findings, pathophysiology, etiology, and gestational age. Surgical therapy, however, consisted irrespective of the classification of a ventricular or lumbar CSF drainage to the right atrium or the peritoneal cavity. Although it was known for a long time that in some forms of “obstructive hydrocephalus” unblocking of the obstruction or passing by of the obstructive site might “cure” the symptoms caused by the hydrocephalus, this only became a part of the standard neurosurgical armamentarium with the introduction of neuroendoscopy as a safe means to achieve this.
The availability of different surgical treatment modalities necessitated the classification of hydrocephalus to be able to select the best treatment option and to compare results. Because an endoscopic third ventriculocisternostomy (ETV) is only successful in the treatment of “obstructive” forms of hydrocephalus, distinguishing communicating from noncommunicating (or obstructive) hydrocephalus seems warranted.
No consensus exists on what imaging is needed to be able to predict a good result of an ETV or to be able to perform this procedure safely. We will discuss the role of hydrocephalus classification and the different imaging modalities that influence ETV results.
Diagnostics in hydrocephalus
The first step is, of course, the taking of a thorough medical history and performing a complete physical (neurological) examination. Some clues about the etiology of the hydrocephalus may be derived from the medical history and typical signs and symptoms at the exam may indicate the presence of a hydrocephalus. However, the mainstay of the diagnostic process is some means of visualizing the ventricles of the brain and the flow of CSF.
Ultrasonography (US) may be used to explore the contents of the skull in the first 12 to 18 months of life, although lower frequency transducers may be used for penetration through bone at later ages. Its advantage is that it can be a bedside test and can therefore be easily used for screening. The size and shape of the ventricular system can be visualized. Clues on the etiology of the hydrocephalus, e.g., hematomas or tumors, may be found. However, ultrasound is less able to show details of the posterior fossa and is hardly reliable in showing the aqueduct, the floor of the third ventricle or the foramen magnum. Nevertheless, Warf [79] found, in a large series of patients with hydrocephalus, a statistically significant correlation between fourth ventricle size as measured with US and the status of the aqueduct (obstructed or patent during ventriculoscopy).
The quality of the US image is highly investigator-dependent and less reliable in reproducing the image. The best indication for US is follow-up of ventricular dilatation before or after surgical treatment in the first 2 years of life, but in case treatment of ventriculomegaly is indicated, other means of brain imaging are required. Thee-dimensional ultrasonography (3D-US) combined with power Doppler US is able to reveal more details of anatomy and intracranial vascular structures, even enabling virtual neuroendoscopy [36]. Also, the 3D-US allows quicker and observer-independent data acquisition and the possibility of offline analysis, which is not the case with 2D-US [73].
Computerized tomography (CT) is the best means to offer easily available, fast, reliable, and reproducible imaging of the ventricles of the brain. Different investigations can be easily compared. Children often do not have to be sedated. Therefore, CT is ideal for follow-up of ventriculomegaly or its treatment after 1–2 years of age and especially so in an emergency setting. CT may allow differentiation between different forms of hydrocephalus and differentiation of etiological factors, but not in sufficient detail. In occlusive hydrocephalus due to aqueductal stenosis, the fourth ventricle may be relatively small in comparison to the other ventricles. The size of the fourth ventricle, however, is not a very reliable sign in differentiating occlusive hydrocephalus from communicating hydrocephalus. For example, in case of a dilated fourth ventricle, one cannot differentiate between communicating hydrocephalus and fourth ventricle outlet obstruction due to congenital occlusions or to secondary infectious or hemorrhagic processes [17, 38].
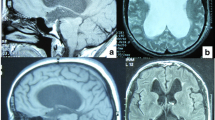
Magnetic resonance imaging (MRI) reveals the most details of the brain and CSF pathways. It allows discrimination of obstructions and membranes, excavations of the ventricles, displacement of anatomical structures, anatomical variations, space-occupying lesions, etc. The constructive interference in steady-state sequence may especially help in delineating membranes and septa in CSF-containing spaces. Besides this, MRI is able to provide qualitative and quantitative information of CSF flow and dynamics [16, 40, 41, 45, 48, 52, 67]. The MRI study of CSF flow in and around the fourth ventricle can be more difficult because the fourth ventricle has three outlets [38]. On the other hand, MRI is usually not readily available, is more expensive, and often requires sedation or even narcosis of the child. Therefore, MRI is not ideal for follow-up diagnostics, but is best used for pretreatment classification of the hydrocephalus.
Lumbar puncture with measurement of intrathecal pressure may help in differentiating communicating from occlusive forms of hydrocephalus but is often not conclusive. The normal range for lumbar CSF opening pressure measured in the flexed lateral decubitus position in children varies between 10 to 28 cm of H2O, which makes it not easy to interpret the test result [20]. Brain imaging (CT or MRI) is required before a lumbar puncture is performed to exclude space-occupying lesions that may cause hindbrain herniation if CSF is derived. Lab investigation of the CSF may shed light on etiological factors, such as infections or hemorrhages that may cause hydrocephalus.
With a lumbar infusion test, the CSF resorption capacity can be assessed, whereas with an intraventricular infusion test, the presence of any structural blockage of CSF circulation between ventricles and the subarachnoid compartment can be assessed. These invasive diagnostic tests may therefore help to differentiate between the communicating and obstructive type of hydrocephalus [3]. The usefulness of these tests and their side effects, however, have not been tested in larger patient groups.
If air or a contrast medium is introduced during lumbar puncture followed by X-ray or CT (pneumoencephalography or intrathecal contrast-enhanced cisternography), the ventricles can be imaged by retrograde filling unless obstructions exist. This may also aid in differentiating between occlusive and communicating hydrocephalus [22, 49]. However, this technique is invasive and may cause significant side effects [28]. In case MRI is not available, one may divert to this option. Intraoperative ventriculography during an endoscopic procedure may also confirm the obstructive nature of a hydrocephalus, as was shown by Karachi et al. [38] in two patients with idiopathic stenosis of the foramina of Magendie and Luschka.
Ventriculoscopy preceding ETV can even be a diagnostic tool! In the absence of CT and MRI, Warf [79] conducted ultrasound studies in hydrocephalus patients in Uganda followed by ventriculoscopy. In 24% of the patients, he found a distorted anatomy, turbid CSF, or congenital anomalies that lead to the abandonment of an ETV attempt.
The latest technique that can be used in the diagnostic process is virtual reality. Rohde et al. [57] described the use of virtual MRI endoscopy with which anomalies of the ventricular anatomy can be studied and its potential role in deciding whether an ETV can be performed safely.
Treatment of hydrocephalus
In those cases where the cause of the hydrocephalus cannot be treated, hydrocephalus can be surgically treated by either diverting the CSF flow to another cavity (peritoneal space, pleural space, and gallbladder) or into the venous system (atrium of the heart, superior sagittal sinus, or transverse sinus), for which a corpus alienum is required, or by so-called internal shunting for which in most cases no corpus alienum is needed. Different techniques of internal shunting were described: third ventriculostomy through subtemporal craniotomy [14], the Torkildsen ETV [76, 77], microsurgical ETV [53, 63], stereotactic ETV [13, 33, 62], fluoroscopic ETV [30, 32], microsurgical opening of the lamina terminalis [64], reconstruction of or stent placement in the aqueduct [44], and endoscopic aqueductoplasty [66]. Nevertheless, ventriculoperitoneal (VP) shunting was the method of choice in the last few decades because it had the lowest complication rate and was the most reliable and most versatile. The etiology and classification of the hydrocephalus did not play a role in the decision to perform a shunting procedure. In the nineties, however, more and more articles reported of the success of ETV in the treatment of obstructive hydrocephalus with low morbidity and complication rates. A major difference to a shunting procedure was that the ETV success rate is dependent of hydrocephalus etiology and classification. The problem rose that in certain situations alternative therapies could be offered to the patient, whereas no evidence toward the superiority of either procedure existed. To date, it has not been possible to perform a prospective randomized study comparing both procedures for the fact that surgeons are so convinced of the superiority of ETV for certain indications that they are not willing to randomly assign their patients to either procedure. A Dutch prospective multicenter study randomizing patients blindly to either procedure had to be aborted because of this.
Garton et al. [25] could not find a significant difference between patients who underwent ETV vs a matched cohort of retrospectively identified patients with a CSF shunt in costs or effectiveness of treatment.
Recently, a new effort was made to resolve the debate of ETV vs shunting by initiating a new prospective randomized controlled trial of ETV vs shunting in children presenting under the age of 2 years with pure aqueduct stenosis. This is the International Infant Hydrocephalus Study, which was presented on the 32nd Annual Meeting of the International Society for Pediatric Neurosurgery in 2004 [68].
Significant differences exist between a shunting procedure and an ETV. Whereas the shunting procedure is relatively simple and easy to learn and therefore often performed by the youngest resident, an ETV is technically more demanding and therefore performed by more experienced neurosurgeons. A shunt placement has always been part of neurosurgical training worldwide and routine neurosurgery. On the contrary, ETV in most countries and institutions is performed by specialized neurosurgeons who perform endoscopic neurosurgery and is not part of routine neurosurgery. The former has a very low, almost negligible intraoperative or perioperative complication rate, whereas during the latter procedure, significant and potentially life-threatening complications may occur with a mortality of 0–2%, major complications in 3.3–12.1%, permanent morbidity in 0–3.6%, and transient morbidity in 3.3–14.9% [1, 5, 7, 18, 25, 34, 51, 65, 70, 71, 79]. Increasing experience with ETV and a high volume of cases, however, seem to decrease the complication rate significantly [65]. On the other hand, if performed successfully, the ETV has few late postoperative complications, in contrast to the shunt operation, after which complications (other than mechanical obstruction) occur in 10.9–29% of cases, the majority of which is a shunt infection [19, 39, 47, 78]. Shunt infection rates vary from 0.33 to 19.6% [8, 10, 12, 15, 39, 46, 50, 54, 81]. Mechanical complications in shunts including late shunt malfunction may even occur in up to 45.9% in the first postoperative year and up to 81% of patients at 12 years follow-up [9, 39, 61]. In normal pressure hydrocephalus the postoperative complication rate of a VP shunt may even be as high as 38% [24]. In lumboperitoneal shunts the revision rate varies extremely from 14 to 86% with an infection rate varying from 1 to 33% [1, 37, 80]. Also, the long-term shunt-related mortality rate of shunt-treated patients, despite many improvements in shunt technique, diagnostics, and follow-up, remains high (2.9 to 12.4% at 10 years follow-up) [46, 69, 78].
A shunt is practically always successful in relieving intracranial pressure caused by hydrocephalus, but the success of ETV has to be awaited for days or even weeks. The success of a shunt, however, is not endurable. Many shunts have to be revised sooner or later. A successful ETV most often is a guarantee for a long-lasting result, although some cases of late hydrocephalus recurrence, even after 6 years, were reported [5, 11, 23, 71].
The effect of a CSF shunt can be easily assessed by ultrasound, CT, or MRI in which a decrease in ventricular size can be objectivated. In contrast, after ETV the ventricular size decreases only slightly in many patients and even remains unchanged in a significant proportion of the patients (up to 42% 1 year after ETV in the series of Fukuhara et al. [23]). In such case, one can only rely on clinical symptoms and signs when evaluating the result of ETV.
All these differences play a role in the decision-making process whether to perform an ETV or place a shunt. While a shunt is a “one-size-fits-all” kind of treatment (more or less), the ETV is only successful in certain subgroups of patients. Success rates of ETV were reported as 50–88% in aqueductal stenosis, 60–95% in posterior fossa tumors, 21–72% in myelomeningocele patients, 22–81% after meningitis/infection, 44–71% in posthemorrhagic hydrocephalus, 52–82% in overall obstructive hydrocephalus, 72% in normal pressure hydrocephalus, and 25–67% in hydrocephalus of unknown etiology [2, 4–7, 18, 21, 23–25, 29, 34, 35, 43, 58, 60, 70, 72, 74, 75, 79]. Results of ETV seem to be lower in young infants, although others dispute this [26, 29, 42, 79]. The lower results in the very young seem to reflect the different etiologies in this age group compared to older patients [4, 26, 79]. Even in repeat ETVs, success rates of 38–65% were reported [71, 79].
Therefore, classification of hydrocephalus becomes critical in predicting the result of an ETV. This requires more details of the hydrocephalus than only the presence of a dilated ventricular system with corresponding signs and symptoms, which might be sufficient to adequately perform a shunting procedure. The single most valuable investigation to obtain maximum information is MRI. Besides anatomical information it also may give information on CSF flow dynamics. No other investigation or combination of investigations will give as much detail as MRI. Nevertheless, additional investigations may add information to the MRI.
Fourth ventricle outflow obstruction, a condition than can be successfully treated by ETV, can be difficult to assess by MRI alone. In those cases, where fourth-ventricle outflow obstruction is anticipated or discussed, intrathecal contrast examination followed by CT scan might be helpful.
Technical considerations
Detailed knowledge of the patient’s brain anatomy is not required to safely place a ventricular drain in a ventricle, although the anatomy of the ventricles should be known. In contrast, a successful ETV is highly dependent on a thorough study of a patient’s individual anatomy [57]. Anomalies and variants of anatomical landmarks for intraventricular endoscopy do occur in a significant number of the patients (36% in the series of Rohde et al. [57]) [7, 56].
The minimum information that should be derived from imaging studies for an optimal procedure planning is listed in Table 1. A correct position of skin incision and burr hole is dependent of the relationship between foramen of Monro and the premamillary floor of the third ventricle because a straight trajectory should be attempted. The size and type (rigid or flexible) of the preferred endoscope is dependent of the ventricular and foramen of Monro diameters. In case the floor of the third ventricle is not translucent, the position of the anatomical structures and relationships beyond it should be known in advance.
Space-occupying lesions may significantly distort the anatomy. Posterior fossa tumors especially displace the mesencephalon and pons anteriorly and cranially, thus reducing the space between brainstem/basilar artery and dorsum sellae and increasing the slope of the third ventricle floor.
Patients with a myelomeningocele and consecutive hydrocephalus, which is often of the obstructive kind, have an aberrant anatomy of the anatomy of the third ventricle [79]. The interthalamic adhesion is mostly relatively large and thus obstructs the pathway of the endoscope. The anterior skull base is relatively short with a dorsum sellae in a relative anterior position, requiring a more posterior burr hole. Also, behind the mamillary bodies, a translucent or thinned part of the ventricular floor often exists that might be held for the tuber cinereum. If one is not aware of the different anatomy, one may perforate the floor behind the basilar artery with possible damage to the perforating arteries.
In severe congenital hydrocephalus with very large ventricles monoventricles often exists and anatomy may be so distorted that only few landmarks exist. In such cases one may profit from image guidance during the procedure, i.e., navigated endoscopy, preferably with electromagnetic (EM) field navigation. With EM navigation, head fixation is not needed, which suits ideally for small children. Relatively small ventricles (e.g., ETV for shunt dysfunction or ETV in the treatment of slit ventricle syndrome) and multiple targets during endoscopy (e.g., ETV combined with endoscopic tumor biopsy) are also good indications for the use of MRI-based EM-tracked endoscope navigation. In those cases of obstructing hydrocephalus with small ventricles and/or distorted anatomy, we will also consider microsurgical III ventriculostomy through the lamina terminalis together with fenestration of Liliequist’s membrane by a supraorbital approach as an alternative to ETV.
Surgical philosophy and ETV
Because there is no evidence concerning which is the best treatment for specific cases of hydrocephalus, it is up to the neurosurgeon to choose the best treatment for each patient. This is a very subjective matter and dependent on training, experience, endoscopy caseload, complications that have occurred in the past during endoscopic procedures or that were observed or taken notice of, number of patients with hydrocephalus annually, and also on the character of the neurosurgeon.
If the caseload of endoscopic procedures is low or once one has had some serious complications during an endoscopic procedure, one will earlier choose for a drain. On the other hand, a consecutive series of successful ETVs without complications will stretch the indication for ETV toward those cases where an obstructive etiology seems less likely. This approach is very subjective and is not supported by any reliable scientific data.
A neurosurgeon who is familiar with the endoscopic technique and feels safe performing an ETV might think of an ETV as “if it doesn’t help, it doesn’t hurt either.” With such a philosophy, it becomes less important to know the etiology of a hydrocephalus because an ETV is tried anyway. This philosophy is supported by the fact that even in cases where a communicating hydrocephalus is suspected (e.g., after infections or intraventricular hemorrhages), the ETV can be successful without the need of a permanent drain [72, 79]. Grant and McLone [27] pointed out that “any patient with a hydrocephalus is a candidate for this procedure.” Sainte-Rose and Chumas [59] identified “the desire to leave a patient shunt free as being a powerful inducement to try the procedure in less than ideal patients.”
In a very recent study Warf presented his results of ETV as the initial treatment of hydrocephalus in a large series of hydrocephalus in Uganda in which 81% of the patients was younger than 1 year of age [79]. In a developing country like Uganda, shunts may not be available and even when available most patients cannot afford such a device. Also, shunt-dependency is a danger in itself for a patient in a developing country. In such a setting ETV as a primary procedure for all cases with minimal diagnostics (only ultrasound in Warf’s series) is a sound rationale and is supported by the excellent results that have been obtained.
However, morbidity of ETV, albeit low, can be significant if it occurs with permanent damage and this should therefore be considered when offering a surgical procedure with a probable low success rate.
If a neurosurgeon is favoring this surgical strategy, less detail from preoperative diagnostics is required because the etiology is not or less playing a role in the decision-making process. Nevertheless, only detailed information of the patient’s individual anatomy can make the ETV a safe surgical procedure. Only MRI can give this information in such detail and therefore MRI does not loose importance with this strategy.
Only few neurosurgeons, however, advocate this philosophy and with increasing experience of the neurosurgical community with ETV as an alternative treatment for hydrocephalus, the indications are getting narrowed down toward clear obstructive hydrocephalus or those indications for which reliable data and results of ETV exist [7, 31].
Elective vs nonelective surgery
It is not always possible to perform optimal diagnostic tests before treatment of hydrocephalus. A rapid decline of the patient’s neurological status may require acute surgical intervention. A MRI may not be available at odd hours or an anesthetized patient may cause logistic problems in planning a MRI. In the newborn this situation hardly ever occurs, as a result of open skull sutures, and the neurosurgeon has enough time to acquire all necessary information. The postnatal-acquired acute hydrocephalus can be a result of meningitis, and is then treated by external ventricular drainage, or the result of a hemorrhage or a tumor leading to an obstructive hydrocephalus. Therefore, an acquired, acutely presenting, newly diagnosed hydrocephalus is, in the majority of the cases, the result of an obstruction and not of malresorption. A CT is usually enough to confirm the diagnosis of hydrocephalus and show the probable cause of the obstruction as well. To be able to predict the result of an ETV a MRI is therefore less indicated in those cases. It is also likely that the anatomy of the patient’s brain and ventricles is not abnormal. However, expanding lesions such as hematomas and posterior fossa tumors might distort the anatomy, e.g., by pushing up the brainstem or obliterating the space between basilar artery and dorsum sellae. Understanding the nature of the lesion helps to predict the anatomic distortion that occurs so that the experienced neurosurgeon is aware of possible distortions without having them visualized by diagnostic tools and thus one can anticipate the situation and perform an ETV safely without extra detailed MRI-derived anatomical information.
Nevertheless, optimization of anatomical and pathophysiological information before performing an ETV should be strived at.
The most frequent emergency case, however, is not the newly diagnosed patient with hydrocephalus but the well-known patient with a drain dysfunction. In those patients the original nature of the hydrocephalus should be already known and previous diagnostics will (or should) be available. If the patient is of the pre-ETV era (at least in your hospital) and is known to have an obstructive hydrocephalus, one should consider performing an ETV and removing the drain (with or without an escape external ventricular or lumbar drainage). If the etiology is unclear, one should consider postponing definite surgery by performing a lumbar tap or CSF reservoir tap and thus buying time for further (MR) imaging.
Informed consent
In former days, when a patient consulted a doctor, he or she accepted the doctor’s opinion and advice without questioning. Nowadays, the doctor is obliged to inform the patient in such a manner about his or her condition and the treatment options that the patient should be able to take a well-considered and autonomous decision regarding treatment: informed consent.
With the emergence of patient interest groups and Internet, a lot of information on hydrocephalus and its treatment has become readily accessible to patients and/or their caregivers. Patients may therefore be well prepared before consulting a doctor or will seek further information after having been consulted.
In the case of hydrocephalus, informed consent and the well-informed patient (or caregivers) necessitate the neurosurgeon to offer all information available on the surgical treatment options, the pro and cons of different strategies, the risks and complication rates, and the success rates of each procedure. To be able to deliver this information tailored to the patient’s individual situation, optimal diagnostics should be available. MRI is thus inevitable as a tool to obtain the necessary information.
Conclusion
There is still no evidence comparing the results and safety of shunts vs ETV and neurosurgeons can therefore choose different strategies for diagnosing and treating hydrocephalus. However, in case ETV is part of the neurosurgeon’s armamentarium, and the neurosurgeon is willing to give a state-of-the-art surgical procedure, the MRI should be considered as a conditio sine qua non.
References
Aoki N (1990) Lumboperitoneal shunt: clinical applications, complications, and comparison with ventriculoperitoneal shunt. Neurosurgery 26:998–1003
Aquilina K, Edwards RJ, Pople IK (2003) Routine placement of a ventricular reservoir at endoscopic third ventriculostomy. Neurosurgery 53:91–96
Bech RA, Bogeskov L, Borgesen SE, Juhler M (1999) Indications for shunt insertion or III ventriculostomy in hydrocephalic children, guided by lumbar and intraventricular infusion tests. Childs Nerv Syst 15:213–217
Beems T, Grotenhuis JA (2002) Is the success rate of endoscopic third ventriculostomy age-dependent? An analysis of the results of endoscopic third ventriculostomy in young children. Childs Nerv Syst 18:605–608
Beems T, Grotenhuis JA (2004) Long-term complications and definition of failure of neuroendoscopic procedures. Childs Nerv Syst 20:868–877
Boschert J, Hellwig D, Krauss JK (2003) Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg 98:1032–1039
Brockmeyer D, Abtin K, Carey L, Walker ML (1998) Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg 28:236–240
Bruinsma N, Stobberingh EE, Herpers MJ, Vles JS, Weber BJ, Gavilanes DA (2000) Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect 6:202–206
Caldarelli M, Di Rocco C, La Marca F (1996) Shunt complications in the first postoperative year in children with meningomyelocele. Childs Nerv Syst 12: 748–754
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77:875–880
Cinalli G, Sainte-Rose C, Chumas P, Zerah M, Brunelle F, Lot G, Pierre-Kahn A, Renier D (1999) Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. J Neurosurg 90:448–454
Cochrane DD, Kestle JR (2003) The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg 38:295–301
Dalrymple SJ, Kelly PJ (1992) Computer-assisted stereotactic third ventriculostomy in the management of noncommunicating hydrocephalus. Stereotact Funct Neurosurg 59:105–110
Dandy W (1922) Treatment of non-encapsulated brain tumors by extensive resection of contiguous brain tissue. IV. An operative procedure for hydrocephalus. Johns Hopkins Hosp Bull 33:189–190
Davis SE, Levy ML, McComb JG, Masri-Lavine L (1999) Does age or other factors influence the incidence of ventriculoperitoneal shunt infections? Pediatr Neurosurg 30:253–257
de Marco G, Idy-Peretti I, Didon-Poncelet A, Baledent O, Onen F, Feugeas MC (2004) Intracranial fluid dynamics in normal and hydrocephalic states: systems analysis with phase-contrast magnetic resonance imaging. J Comput Assist Tomogr 28:247–254
Decq P, Le Guerinel C, Sol JC, Brugieres P, Djindjian M, Nguyen JP (2001) Chiari I malformation: a rare cause of noncommunicating hydrocephalus treated by third ventriculostomy. J Neurosurg 95:783–790
Devaux BC, Joly LM, Page P, Nataf F, Turak B, Beuvon F, Trystram D, Roux FX (2004) Laser-assisted endoscopic third ventriculostomy for obstructive hydrocephalus: technique and results in a series of 40 consecutive cases. Lasers Surg Med 34:368–378
Di Rocco C, Marchese E, Velardi F (1994) A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst 10:321–327
Ellis R III (1994) Lumbar cerebrospinal fluid opening pressure measured in a flexed lateral decubitus position in children. Pediatrics 93:622–623
Feng H, Huang G, Liao X, Fu K, Tan H, Pu H, Cheng Y, Liu W, Zhao D (2004) Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg 100:626–633
Figaji AA, Fieggen AG, Peter JC (2004) Air encephalography for hydrocephalus in the era of neuroendoscopy. Childs Nerv Syst 21(7):559–565
Fukuhara T, Vorster SJ, Luciano MG (2000) Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery 46:1100–1109
Gangemi M, Maiuri F, Buonamassa S, Colella G, de Divitiis E (2004) Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus. Neurosurgery 55:129–134
Garton HJ, Kestle JR, Cochrane DD, Steinbok P (2002) A cost-effectiveness analysis of endoscopic third ventriculostomy. Neurosurgery 51:69–77
Gorayeb RP, Cavalheiro S, Zymberg ST (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg Spine 100:427–429
Grant JA, McLone DG (1997) Third ventriculostomy: a review. Surg Neurol 47:210–212
Greenberger R, Khangure MS, Chakera TM (1987) The morbidity of CT air meatography: a follow-up of 84 patients. Clin Radiol 38:535–536
Grunert P, Charalampaki P, Hopf N, Filippi R (2003) The role of third ventriculostomy in the management of obstructive hydrocephalus. Minim Invasive Neurosurg 46:16–21
Guiot G (1973) Ventriculo-cisternostomy for stenosis of the aqueduct of Sylvius. Puncture of the floor of the third ventricle with a leucotome under television control. Acta Neurochir (Wien) 28:275–289
Hellwig D, Grotenhuis JA, Tirakotai W, Riegel T, Schulte DM, Bauer BL, Bertalanffy H (2005) Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev 28:1–34
Hirsch JF, Hirsch E, Sainte RC, Renier D, Pierre-Khan A (1986) Stenosis of the aqueduct of Sylvius. Etiology and treatment. J Neurosurg Sci 30:29–39
Hoffman HJ, Harwood-Nash D, Gilday DL (1980) Percutaneous third ventriculostomy in the management of noncommunicating hydrocephalus. Neurosurgery 7:313–321
Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A (1999) Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery 44:795–804
Javadpour M, Mallucci C (2004) The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst 20:852–857
Jodicke A, Berthold LD, Scharbrodt W, Schroth I, Reiss I, Neubauer BA, Boker DK (2003) Endoscopic surgical anatomy of the paediatric third ventricle studied using virtual neuroendoscopy based on 3-D ultrasonography. Childs Nerv Syst 19:325–331
Karabatsou K, Quigley G, Buxton N, Foy P, Mallucci C (2004) Lumboperitoneal shunts: are the complications acceptable? Acta Neurochir (Wien) 146:1193–1197
Karachi C, Le Guerinel C, Brugieres P, Melon E, Decq P (2003) Hydrocephalus due to idiopathic stenosis of the foramina of Magendie and Luschka. Report of three cases. J Neurosurg 98:897–902
Kay AD, Fisher AJ, O’Kane C, Richards HK, Pickard JD (2000) A clinical audit of the Hakim programmable valve in patients with complex hydrocephalus. Br J Neurosurg 14:535–542
Kim DS, Choi JU, Huh R, Yun PH, Kim DI (1999) Quantitative assessment of cerebrospinal fluid hydrodynamics using a phase-contrast cine MR image in hydrocephalus. Childs Nerv Syst 15:461–467
Kim MH, Shin KM, Song JH (1998) Cine MR CSF flow study in hydrocephalus: what are the valuable parameters? Acta Neurochir Suppl 71:343–346
Koch D, Wagner W (2004) Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome? Childs Nerv Syst 20:405–411
Kulkarni AV, Drake JM, Armstrong DC, Dirks PB (2000) Imaging correlates of successful endoscopic third ventriculostomy. J Neurosurg 92:915–919
Leksell L (1949) A surgical procedure for atresia of the aqueduct of Sylvius. Acta Psychiatr Neurol Scand 24:559–568
Mascalchi M, Arnetoli G, Inzitari D, Dal Pozzo G, Lolli F, Caramella D, Bartolozzi C (1993) Cine-MR imaging of aqueductal CSF flow in normal pressure hydrocephalus syndrome before and after CSF shunt. Acta Radiol 34:586–592
Mazza C, Pasqualin A, Da Pian R (1980) Results of treatment with ventriculoatrial and ventriculoperitoneal shunt in infantile nontumoral hydrocephalus. Childs Brain 7:1–14
Meier U (2003) Shunt operation versus endoscopic ventriculostomy in normal pressure hydrocephalus: diagnostics and outcome. Zentralbl Neurochir 64:19–23
Mohanty A, Vasudev MK, Sampath S, Radhesh S, Sastry Kolluri VR (2002) Failed endoscopic third ventriculostomy in children: management options. Pediatr Neurosurg 37:304–309
Palma L, Mariottini A, D’Addetta R, Mastronardi L (1988) RISA cisternography in the option of ventriculocisternal shunt for infantile non-tumoural aqueductal stenosis. Acta Neurochir Suppl (Wien) 42:225–229
Piatt JH Jr, Carlson CV (1993) A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg 19:233–241
Pople IK, Edwards RJ, Aquilina K (2001) Endoscopic methods of hydrocephalus treatment. Neurosurg Clin N Am 12:719–735, viii
Quencer RM (1992) Intracranial CSF flow in pediatric hydrocephalus: evaluation with cine-MR imaging. Am J Neuroradiol 13:601–608
Reddy K, Fewer HD, West M, Hill NC (1988) Slit ventricle syndrome with aqueduct stenosis: third ventriculostomy as definitive treatment. Neurosurgery 23:756–759
Reinprecht A, Dietrich W, Berger A, Bavinzski G, Weninger M, Czech T (2001) Posthemorrhagic hydrocephalus in preterm infants: long-term follow-up and shunt-related complications. Childs Nerv Syst 17:663–669
Rekate HL (2001) Hydrocephalus: classification and pathophysiology. In: McLone DG (ed) Pediatric neurosurgery: surgery of the developing nervous system. Saunders, Philadelphia, pp 457–474
Rohde V, Gilsbach JM (2000) Anomalies and variants of the endoscopic anatomy for third ventriculostomy. Minim Invasive Neurosurg 43:111–117
Rohde V, Krombach GA, Struffert T, Gilsbach JM (2001) Virtual MRI endoscopy: detection of anomalies of the ventricular anatomy and its possible role as a presurgical planning tool for endoscopic third ventriculostomy. Acta Neurochir (Wien) 143:1085–1091
Ruggiero C, Cinalli G, Spennato P, Aliberti F, Cianciulli E, Trischitta V, Maggi G (2004) Endoscopic third ventriculostomy in the treatment of hydrocephalus in posterior fossa tumors in children. Childs Nerv Syst 20:828–833
Sainte-Rose C, Chumas P (1996) Endoscopic third ventriculostomy. Tech Neurosurg 1:176–184
Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, Carpentier A, Bourgeois M, Zerah M, Pierre-Kahn A, Renier D (2001) Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 95:791–797
Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ, Humphreys RP, Hendrick EB (1991) Mechanical complications in shunts. Pediatr Neurosurg 17:2–9
Sayers MP, Kosnik EJ (1976) Percutaneous third ventriculostomy: experience and technique. Childs Brain 2:24–30
Scarff JE (1935) Third ventriculoscopy as the rational treatment of obstructive hydrocephalus. J Pediatr 6:870–871
Scarff JE (1966) Third ventriculostomy by puncture of the lamina terminalis and the floor of third ventricle. J Neurosurg 24:935–943
Schroeder HW, Niendorf WR, Gaab MR (2002) Complications of endoscopic third ventriculostomy. J Neurosurg 96:1032–1040
Schroeder HW, Oertel J, Gaab MR (2004) Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst 20:821–827
Schroeder HW, Schweim C, Schweim KH, Gaab MR (2000) Analysis of aqueductal cerebrospinal fluid flow after endoscopic aqueductoplasty by using cine phase-contrast magnetic resonance imaging. J Neurosurg 93:237–244
Sgouros S, Kulkarni AV, Constantini S (2004) International infant hydrocephalus study: rational and scope of the trial. Childs Nerv Syst 20(8–9):664 (Abstract)
Sgouros S, Malluci C, Walsh AR, Hockley AD (1995) Long-term complications of hydrocephalus. Pediatr Neurosurg 23:127–132
Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, Weiner H, Roth J, Beni-Adani L, Pierre-Kahn A, Takahashi Y, Mallucci C, Abbott R, Wisoff J, Constantini S (2002) Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg 97:519–524
Siomin V, Weiner H, Wisoff J, Cinalli G, Pierre-Kahn A, Saint-Rose C, Abbott R, Elran H, Beni-Adani L, Ouaknine G, Constantini S (2001) Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst 17:551–555
Smyth MD, Tubbs RS, Wellons JC, III, Oakes WJ, Blount JP, Grabb PA (2003) Endoscopic third ventriculostomy for hydrocephalus secondary to central nervous system infection or intraventricular hemorrhage in children. Pediatr Neurosurg 39:258–263
Stanojevic M, Hafner T, Kurjak A (2002) Three-dimensional (3D) ultrasound—a useful imaging technique in the assessment of neonatal brain. J Perinat Med 30:74–83
Teo C, Jones R (1996) Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg 25:57–63
Tisell M, Almstrom O, Stephensen H, Tullberg M, Wikkelso C (2000) How effective is endoscopic third ventriculostomy in treating adult hydrocephalus caused by primary aqueductal stenosis? Neurosurgery 46:104–110
Torkildsen A (1939) New palliative operation in cases of inoperable occlusion of the Sylvian aqueduct. Acta Psychiatr Neurol Scand 82:117–123
Torkildsen A (1960) A follow-up study 14 to 20 years after ventriculocisternostomy. Acta Psychiatr Scand 35:113–121
Tuli S, Tuli J, Drake J, Spears J (2004) Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg: Pediatrics 100:442–446
Warf BC (2005) Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg 102(Suppl 1):1–15
Yadav YR, Pande S, Raina VK, Singh M (2004) Lumboperitoneal shunts: review of 409 cases. Neurol India 52:188–190
Zemack G, Romner B (2000) Seven years of clinical experience with the programmable Codman Hakim valve: a retrospective study of 583 patients. J Neurosurg 92:941–948
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Lindert, E.J., Beems, T. & Grotenhuis, J.A. The role of different imaging modalities: is MRI a conditio sine qua non for ETV?. Childs Nerv Syst 22, 1529–1536 (2006). https://doi.org/10.1007/s00381-006-0189-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0189-1