Abstract
Background and purpose
The ability of cranial bone to repair defects of continuity is limited and it is mostly dependent on the age of the patient. In infancy and in early pediatric age, the scarce thickness of the calvarial bones and the need for a harmonic development of the child’s skull limit the application of most of the surgical procedures usually utilized in older patients. We tested the ability of mononucleated cells, derived from the patient’s bone marrow and transplanted on the site of the cranial bone defect, to increase the rate of mineralization of the autologous osteogenesis to obtain the complete restoration of the skull continuity.
Method
Four children, aged 26, 28, 37, and 79 months, respectively, affected by a stabilized and persistent cranial bone defect of posttraumatic or postsurgical origin, were treated. A sandwich-shaped shell, made of extrused absorbable polylactic copolymers material, was used to hold in place a freeze-dried mineralized collagen matrix associated with a nonceramic hydroxyapatite scaffold, where autologous bone marrow mononucleated cells were inseminated.
Results
In all patients, a rapid autologous bone osteogenesis was observed with a clear dimensional reduction of the bone defect few months after the autologous bone marrow cells seeding.
Conclusions
The preliminary results of this research suggest the use of autologous bone marrow cells to increase the autologous osteogenesis in early pediatric age in cases in which correction of skull bone defects is best realized with autologous bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repairing of posttraumatic skull defects and/or congenital morphological craniofacial anomalies may require complex surgical procedures during which wide bone surfaces need to be removed, mobilized, remodeled, or integrated. The ability of cranial bone to repair defects of continuity is limited and it is mostly dependent on the age of the patient, being close to zero in adults. In infants the osteoreparative potential is high and sufficient to repair. The scarce thickness of the skull during infancy does not allow any splitting, consequently preventing the correction of those cases where extensive amounts of bone are usually required.
Moreover, according to the metabolic dynamics guiding tissue repair, in infants as well as in adults, the biological priority expressed in the scarring process privileges the lowest complexity cellular lines, i.e., mainly fibrotic tissue. Consequently, in the specific case of skull bone defects, the repair goes on toward low-specialized fibrous scar tissue, when not adequately guided or conditioned. In these cases, production of reparative bone tissue may be poor.
In young patients, an alternative cranial graft made of allogeneic or ceramic inorganic matrices would not guarantee the level of osteointegration needed for the harmonic development of the skull; thus, possibly leading to cranial constrictive deformity, or resulting in dislocation of the graft.
Large sheets of extrused polylactic acid copolymers may be used to repair wide cranial bone defects. This material would protect the brain from external mechanical damages, increase the local osteogenetic activity by maintaining the fibrous scar tissue away from the craniolacunia, completely resorb within 10/14 months; thus, overcoming the risk of skull restrictions, due to limited local bone expansion.
The reliability of the autologous osteogenetic process, however, may be inconstant and only occasionally fast enough to complete the repair of large bone defects within the span time needed for the complete resorption of the polylactic graft.
The aim of the present study is to test the ability of mononucleated cells derived from the patient’s bone marrow and transplanted on the site of the cranial bone defect to increase the rate of mineralization of the autologous osteogenesis as to obtain a complete restoration of the skull continuity within the resorption time of the polylactic bone grafting material.
Materials and methods
Patient’s population
Four children, aged 26, 28, 37, and 79 months, respectively, affected by a stabilized skull bone defect of posttraumatic or postsurgical origin, observed at the Pediatric Neurotraumatology Unit of the “Bambino Gesù” Pediatric Research Hospital, Rome, Italy, between January 2001 and June 2003, were enrolled in the study. In this group of patients, no osteoblastic activity was recorded on the site of the cranial bone defect during the period of observation (23, 22, 15, and 68 months, respectively, mean 31.5 months; range 15–68 months) after the initial surgical event (Table 1).
None of the patients was harboring a skull bone defect associated with a congenital midline anomaly (encephalocele, cranioschisis), nor presented a positive history for skull bone infection.
Imaging work-up
All patients underwent an imaging work-up to define the preoperative morphology of the bone defect precisely. This consisted of an examination with a 3D-reformat representation. In all the cases, the surface rendering reconstruction level was set at 140 HU.
Brain magnetic resonance imaging was obtained to evaluate potential focal postlesional parenchymal damages that could have impaired the normal bone scarring process (i.e., “growing fracture” phenomenon).
Hematologic work-up, bone marrow aspiration, and cell isolation
All patients underwent a routine hematologic work-up. The day of surgery, just before starting the operative procedure for cranioplastic correction and with the patient already under general anesthesia, a mean volume of 105 ml of bone marrow (range 77–125 ml) was aspirated from the iliac crest of the patients. To prevent clotting and embolization, anticoagulation was realized with ACD-A (Baxter Fenwall, USA) and filtration was performed with a transfusion set for blood components (Baxter, USA). Nucleated cells were separated by centrifugation at 1,500 rpm for 15 min and plasma removed. Eritro depletion was obtained by gravitational separation (HES 6%—MW 450.000 d—Plasmasteril–Fresenius Kobi, Germany).
Before insemination, the cellular concentrates were reduced at a mean volume of 6.87 ml (range 5.1–8.3 ml). The mean number of nucleated cells and CD34+ cells inseminated was 17.53×108 (range 6.9–39.8) and 23.19×106 (range 15.53–23.98), respectively.
Surgical treatment
In all patients, the persistent cranial bone defect was treated by applying a sandwich-shaped graft made of two sheets of extrused absorbable polylactic copolymers material [70:30 copolymeric proportion, 0.75 mm thickness, carrying micropores of 450 μm in diameter (original design F. V.), estimated resorption time 14 months, custom made by Macropore & BC—Sofamor Danek—San Diego CA. USA], within which bone marrow derived mononucleated cells were seeded.
At the end of the autologous bone marrow harvesting procedure, the patients underwent the cranioplastic surgical procedure.
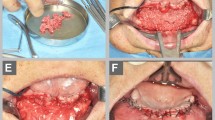
After the incision of the skin, the exposition of the lesion, and the accurate skeletrization of its margins were realized. Specific attention was paid to avoid any dural tear, mostly while dissecting the scarred osteodural interface along the margins of the cranial defect (Fig. 1).
Synopsis of the surgical procedure. Lesion is exposed (a); margins are skeletrized (b); subdural space is dissected and the internal polylactic sheet is inserted (c); scaffold is tailored (d); concentrated mononucleated cells from autologous bone marrow are seeded in the scaffold (e); external polylactic sheet is applied
Once the dura was thoroughly freed, one sheet of plastic material was tailored a few millimeters larger than the cranial bone gap to be filled, tridimensionally shaped, inserted in the epidural space, and hinged all around the margins of the cranial defect (internal sheet).
The harvested autologous bone marrow mononucleated cells were inseminated in a scaffold made of a freeze-dried mineralized collagen matrix associated with a nonceramic hydroxyapatite [Ca10(PO4)6(OH)2]. The scaffold was then accommodated on the internal sheet of polylactic material as to completely fill the bone gap (Fig. 1).
A second sheet of polylactic material, modeled by bending and applying some gore-shaped cuts on its margins, was assembled over the skull in a sandwich fashion. At the end of the procedure, the external sheet of polylactic material was fixed to the living skull with resorbable polylactic screws.
Postoperative follow-up and ossification assessment
All patients underwent the postoperative and follow-up neuroimaging protocol, that included computed tomography (CT) scan of the skull with 3D reformat representation. The parameters for the gray scale surface rendering in the postoperative CT-3D were exactly at the same level (140 HU) with that in the preoperative examinations. This allowed an objective analysis of the radiological results and a reliable definition of the morpho-volumetric changes due to the induced ossification (Figs. 2, 3, and 4).
M. D.: 3D reformatted CT scans with surface rendering reconstruction (level set at 140 HU): a Bone defect was determined in June 2001 at 6 months of age by a traffic accident. b Cranioplasty with autologous bone marrow cells transplant was realized in June 2003. No previous spontaneous osteogenetic activity was observed. c Clear evidence of osteogenetic activity is observed in April 2004, 10 months after the autologous bone marrow cells transplant
A. E.: 3D reformatted CT scans with surface rendering reconstruction (level set at 140 HU): a Cranial bone lesion immediately before (top) and after (bottom) surgery for cranioplasty with autologous bone marrow cells transplant. b Evidence of osteogenetic activity in November 2003, 4 months after surgery. c Eight months after surgery ossification appears almost complete
A. A.: 3D reformatted CT scans with surface rendering reconstruction (level set at 140 HU): a Cranial bone lesion immediately after the post-traumatic craniotomy (August 2001). b Cranial bone lesion two months after surgery for cranioplasty with autologous bone marrow cells transplant (July 2003). c Seven months after cells transplant the novel osteogenetic activity appears evident (February 2004)
Results
In all patients, the surgical procedure was uneventful. The technical procedure showed no special difficulties compared with a typical procedure for bone defect repair using different prosthetic material. Due to the close cooperation with the Department of Haematology, the time that is required to complete the surgical procedure was equivalent to that of a routine case.
Both the double sheet polylactic implant and the freeze-dried mineralized collagen matrix associated with a nonceramic hydroxyapatite scaffold demonstrated to be completely biocompatible.
In all patients, a rapid autologous bone osteogenesis was observed. In patient 4, it was possible to document a clear dimensional reduction of the bone defect as soon as 4 months after the polylactic cranioplastic and autologous bone marrow cells seeding and a complete bone healing after 8 months from the procedure. Larger defects, as those in patients 1 and 2, required a longer osteogenetic reparative activity before the craniolacunia was completely filled by new bone. It is worth to note that in all the cases, even in those harboring the largest bone defects, the mineralizing osteogenetic activity was faster than the metabolic process leading to the resorption of the polylactic double-layer shell. In fact, no clinical evidence, even transient, of softening of the cranioplastic implant was noticed in any of the patients.
Discussion
Cranial bone defects may result from various causes, as congenital faults in development, traumas, or surgical resections, e.g., in the treatment of tumors or infections. The capacity of young children to reossify large calvarial defects has been well established. The regenerative potential of the calvaria is lost in adult animals and humans older than 18–24 months [27, 30]. The use of bone grafts is compromised by limited supply, complications, and unpredictable outcome. Other methods of treatment that have been studied include filling the bone defect with alloplastic materials as hydroxyapatite and bioactive glass [13].
In pediatric patients, allogeneic or ceramic inorganic matrices would not guarantee the level of osteointegration needed for the harmonic development of the child’s skull and would not comply with the physiological morpho-volumetric expansion of the skull (as the living skull bone would have done), would be dislocated, or would determine a site of cranial constrictive deformity.
Novel methods have evolved recently, with the aim of generating or helping to generate autologous bone by way of tissue engineering [19, 26, 35].
One approach in bone tissue engineering involves guided bone regeneration (GBR), in which membranes are used to help bone regeneration in bone defects. The application of the principle of GBR has shown positive results in a number of controlled animal studies [1, 2, 7, 10]. However, a successful healing pattern finalizing the process of bone augmentation (blood cloth formation, invasion by osteoprogenitor cells, their differentiation into osteoblasts, apposition of an extracellular matrix that later mineralizes to form woven bone and later is remodeled into lamellar bone) [12] requires predictable space maintenance and adequate exclusion from the defect area of those cells that lack osteogenetic potential during the healing period. Vesala et al. [36, 37] clearly demonstrated in a rabbit animal model that on the cranial bone, where the healing process is slower than in metaphyseal limb bone, a slowly absorbed material as the polylactide can be more suitable for GBR than faster resorbing polyglicolic membranes. In the cranial site, in fact, polylactic membranes were able to effectively guide the regeneration process in skull bone defects as large as 10 mm of diameter.
In humans, the objective of enhancing the autologous osteogenesis for reconstruction of skull bone defects has been clinically recognized as almost impossible [31].
Cohen et al. [9] suggested in 2004 to overcome this problem realizing a cranioplasty with a double layer of 0.5 polylactic sheets and filling the space in between the sheets with carbonated apatite cement. In their surgical technique, a thin layer of Gelfoam, fitted into the cranial defect between the dura mater and the inner polylactic sheet was used to buffer the dural pulsations, allowing incorporation or fusion of the construct. The authors claim no complications and believe that the use of bioabsorbable materials combined with hydroxiapatite may reduce the long-term complications associated with foreign body implants in the pediatric population, as carbonated apatite may be trusted to induce local osteointegration at the level of the new artificial implant. In pediatric age, however, the peculiar dynamic nature of the skull implies the use of materials complying with the most extended definition of “osteointegration” including the ability to follow the thrusting expansion of the brain parenchyma, as only autologous bone is credited to do.
In our patients, the seeding of autologous mononucleated cells, derived from the patient’s bone marrow and transplanted on the site of the cranial bone defect was taken into consideration, with the aim of increasing the local osteogenetic capacity and repairing the skull bone defect with viable autologous bone. Appropriate material was searched for cell scaffolding.
For clinical use in pediatric patients, the ideal scaffold would be a material presenting with:
-
1.
Good biocompatibility,
-
2.
Reliable osteointegrative properties,
-
3.
Highly interconnected porosity,
-
4.
High mechanical strength,
-
5.
High biodegradability.
As for our knowledge, no such a material is available on the market.
Most of the ceramic scaffolds would present with the first four properties, while none of them is biodegradable. Resorbable ceramic hydroxiapatite, on the contrary, provides properties 1, 2, 3, and 5, but lacks any mechanical strength.
Poly (dl-lactic-co-glycolic) acid polymers, available in porous form, were suggested as ideal scaffolds [32, 33], provided that no load bearing was requested.
The shell made of a double sheet of extrused absorbable polylactic d-l copolymer utilized for our patients, on the contrary, offered such a mechanical strength as to bear sufficient load to allow the use of a synthetic cranial bone flap implant. Our polylactic implant fulfilled requested properties 1, 2, 4, and 5, yet it was not porous at all.
It was reported that the multipotent mesenchymal stem cells contained in the bone marrow are capable of differentiating into osteoblasts, chondrocytes, myoblasts, adipocytes, etc., when adequate scaffold matrices supporting cellular attachment and a stimulating microenvironment is supplied [23].
The soft, porous, and resorbable scaffold, made of a freeze-dried mineralized collagen matrix associated with a nonceramic hydroxyapatite [Ca10(PO4)6(OH)2], in which the autologous bone marrow mononucleated cells were inseminated, provided the honey-comb-like structure, with cells smaller than 500 μm, advocated by Y. Kuboki et al. [20] reported that mesenchymal cells were allowed to show the maximum osteogenetic potential.
The interest on the ability of purified human mesenchymal stem cells derived from normal bone marrow to increase osteogenesis in orthotopic and etherotopic sites was tested in several experimental animal and human works [5, 6, 15, 38].
The bone marrow, in fact, contains a population of rare progenitor cells capable of differentiating into bone, cartilage, tendon, and other connective tissues. The nature and interrelations of the numerous recognized locally effective bioactive proteins, as well as the role of geometrically determined carriers controlling the phenotypic expression in induced osteogenesis and chondrogenesis, have been not completely clarified yet [16, 17, 22, 39]. Some basilar landmarks of the osteoreparative process, however, have been sperimentally defined.
Before new bone formation, proliferation and migration of pluripotent mesenchymal cells originating from periosteum, muscle, or bone marrow stroma are necessary as a source of skeletal tissue cells [14, 34]. Rapid bone formation may occur in areas close to the source of proliferating cells, e.g., periosteum, muscle, or bone marrow. This extraordinary capacity for growth may occur, provided that adhesion of proliferated mesenchymal cells and supply of a series of locally acting diffusible growth and differentiation factors are assured.
The potential of bone marrow as a source of mesenchymal stem cells (marrow stromal cells) has been demonstrated by several investigators [11, 18, 21, 23, 25, 28]. Due to their scarce concentration, however, methods for culture expansion [3–5, 8], growth enhancement, and cellular differentiation have been suggested [16, 22, 24, 29, 31].
The active mineralization showed by our patients at the site of the skull bone defect where the polylactic compound was implanted demonstrates the high potential for growth and osteogenetic differentiation of the nonenhanced, nonstimulated bone marrow cells. All the patients, even those presenting with the largest defects, showed a rapid process of ossification, definitely faster than the resorption process of the polylactic construct. All the bone flaps appeared completely osteointegrated and, at the latest follow-up control, no local or general morbidity was recorded.
Results of this research work promote a simplified protocol suggesting the use of autologous bone marrow cells to induce the autologous osteogenesis in those cases in the early pediatric age in which correction of skull bone defects, deriving from traumatic as well as malformative or neoplastic diseases, are best realized with autologous bone. In our proposed method, all the components of the multilayered construct (both the polylactic and the hydroxyapatite components) dissolve at the end of a metabolic process lasting for several months, leaving in site only viable autologous bone derived from the seeding of an autologous source (bone marrow) within an ingegnerized resorbable implant. The possibility to mold the multilayered polylactic-hydroxyapatite construct in any anatomical form may promote the use of this relatively simple technique to increase the autologous osteogenesis in all those cases in which a complex tridimentionally shaped bony structure has to be reimplanted or remodeled in sites where a relatively low gravitational load is expected (craniofacial regions in primis).
Although the surgical technique required is not different from the one familial to all the surgeons used, in the everyday practice to realize typical cranial repairs with alloplastic materials, and although the procedure required for harvesting and concentrating the bone marrow mononucleated cells is relatively simple, our initial results suggest that the learning curve may be steep at the beginning. Molding of the polylactic sheets at temperatures close to 60/70°C requires experience to avoid polymer denaturation. Cerebrospinal fluid or blood collections within the region of the implanted construct, even as small as to be of no significance in general practice, should be avoided, as they may cause reduced mononucleated cells adhesion and failure of the transplant. Tridimensional CT threshold should be meticulously defined with the Radiology Department to prevent incorrect judgements on load bearing capacity.
References
Ashammakhi N, Mäkelä EA, Vihtonen K (1995) Repair of bone defects with absorbable membranes: an experimental study on rabbits. Ann Chir Gynaecol 84:309–315
Ashammakhi N, Mäkelä EA, Vihtonen K (1994) The effect of self-reinforced polyglycolide membrane on metaphyseal bone: an experimental study on rats. Ann Chir Gynaecol 83:228–234
Bruder SP, Jaiswal N (1996) The osteogenic potential of human mesenchymal stem cells is not diminished after one billion-fold expansion in vitro. Trans Orthop Res Soc 21:580
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self renewed and osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64:278–294
Bruder SP, Kurth AA, Shea M (1998) Bone regeneration by implantation of purified culture expanded human mesenchymal stem cells. J Orthop Res 16:155–162
Bruder SP, Jaiswal N, Ricalton NS (1998) Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res 355S:S247–S256
Buser D, Dula K, Belser UC, Hirt HP, Berthold H (1996) Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg 54:430–432
Caplan AI, Bruder SP (1997) In: Lanza RP, Langer R, Chick WL (eds) Cell and molecular engineering of bone regeneration. Principles of tissue engineering. Academic, New York, pp 603–618
Cohen AJ, Dickerman RD, Schneider SJ (2004) New method of pediatric cranioplasty for skull defect utilizing polylactic acid absorbable plates and carbonated apatite bone cement. J Craniofac Surg 15(3):469–472 (May)
Fiorellini J, EngebretsonS, Donath K, Weber H (1998) Guided bone regeneration utilizing expanded polytetrafluoroethylene membranes in combination with submerged and nonsubmerged dental implants in beagle dogs. J Periodontol 69:528–535
Friedenstein AJ, Piatetzky-Shapiro IJ, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells.
Hämmerle CHF, Chiantella GC, Karring T, Lang NP (1998) The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implants Res 9:151–162
Hench LL (1988) Bioactive ceramics. Ann NY Acad Sci 523:54
Hirano H, Urist MR (1981) Bone-forming and bone-resorbing cell lines derived from bone marrow in tissue culture. Clin Orthop 154:234
Horwitz EM, Prockop DJ, Fitzpatrick LA (1999) Transplantability and therapeutic effect of bone-marrow derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5:309–313
Imai S, Kaksonen M, Raulo E (1998) Osteoblast recruitment and bone formation enhanced by cell matrix-associated heparin-binding growth-associated molecule (HB-GAM). J Cell Biol 143:1113–1128
Itoh S, Kikuchi M, Koyama Y (2002) Development of an artificial vertebral body using a novel biomaterial, hydroxyapatite/collagen composite. Biomaterials 23:3919–3926
Kassem M, Risteli L, Mosekilde L (1991) Formation of osteoblast-like cells from human mononuclear bone marrow cultures. APMIS 99:269–274
Kim Th, Jannetta C, Vacanti JP (1995) Engineered bone from polyglycolic acid polymer scaffold and periosteum. Mater Res Soc Symp Proc 394:91–97
Kuboki Y, Jin Q, Takita H (2001) Geometry of carriers controlling phenotypic Expression in BMP-induced osteogenesis and chondrogenesis. J Bone Jt Surg 83-A(Suppl 1):S1105–S1115
Kuznetsov SA, Krebsbach PH, Satomura K (1997) Single-colony-derived strains of human marrow stromal fibroblasts from bone alter transplantation in vivo. J Bone Miner Res 12:1335–1347
Locoeur L, Ouhayoun JP (1997) In vivo induction of osteogenic differentiation from non-osteogenic mesenchymal cells. Biomaterials 18:989–993
Owen M (1988) Marrow stromal stem cells. J Cell Sci 10(Suppl):63–76
Partridge K, Yang X, Clarke N (2002) Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Comm 292:144–152
Pittenger MF, Mackay AM, Beck SC (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Puelacher WC, Vacanti JP, Ferraro NF (1996) Femoral shaft reconstruction using tissue-engineered growth of bone. Int J Oral Maxillofac Surg 25:223–228
Reedy BK, Pan F, Kim WS, Gannon FH, Krasinskas A, Bartlett SP (1999) Properties of coralline hydroxyapatite and expanded polytetrafluoroethylene membrane in the immature craniofacial skeleton. Plast Reconstr Surg 103:20
Rickard DJ, Kassem M, Hefferan TE (1996) Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res 11:312–332
Ripamonti U, Crooks J, Rueger D (2001) Induction of bone formation by recombinant human osteogenic protein-1 and sintered porous hydroxyapatite in adult pimates. Plast Reconstr Surg 107:977–988
Sirola K (1960) Regeneration of defects in the calvaria. Ann Med Exp Biol Fenn 38(Suppl 2):1
Tabata Y, Yamada K, Miyamoto S (1998) Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials 19:807–815
Thomson RC, Mikos AG, Beahm E (1999) Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials 20:2007–2018
Uemura T, Dong J, Wang Y (2003) Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials 24:2277–2286
Urist MR, Grant TT, Lindholm ST (1979) Induction of new bone formation in the host bed by human bone-tumor transplant in athymic nude mice. J Bone Jt Surg 61A:1207
Vacanti JP, Langer R (1999) Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354(Suppl):S131–S134
Vesala AL, Kallioinen MJ, Vithonen K (2000) Poly-l-lactic acid plate for covering of small cranial bone holes: an experimental study in rabbits. Eur J Plast Surg 23:36–38
Vesala AL, Kallioinen MJ, Törmälä P (2002) Bone tissue engineering: treatment of cranial bone defects in rabbits using self-reinforced poly-l,d-lactide 96/4 sheets. J Craniofac Surg 13:607–613
Vuola J, Göransson H, Böhling T, Asko-Seljavaara S (1996) Bone marrow induced osteogenesis in hydroxiapatite and calcium carbonate implants. Biomaterials 17:1761–1766
Woo BH, Fink BF, Page R (2001) Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm Res 18:1747–1753
Acknowledgements
The research project was approved by the Ethic Committee, Pediatric Research Hospital “Bambino Gesù”, Rome, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velardi, F., Amante, P.R., Caniglia, M. et al. Osteogenesis induced by autologous bone marrow cells transplant in the pediatric skull. Childs Nerv Syst 22, 1158–1166 (2006). https://doi.org/10.1007/s00381-006-0100-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-006-0100-0