Abstract
Case report
We describe a very rare non-infantile case of desmoplastic infantile astrocytoma (DIA). A 9-year-old boy presented with motor weakness and sensory disturbance in his right upper and lower limbs. CT and MRI showed a contrast-enhanced large cystic tumor in the left sensorimotor area. We successfully resected the entire tumor. Its histopathological features were typical of DIA.
Outcome
The patient’s neurological symptoms improved postoperatively. Neither radiotherapy nor chemotherapy was used postoperatively. The patient developed normally and had been doing well for 12 months after surgery without tumor recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Desmoplastic infantile astrocytoma (DIA) is a rare brain tumor that presents as a large hemispheric mass in infants. It has a biphasic histologic pattern consisting of glial and mesenchymal elements. Despite an ominous histologic picture that may resemble a sarcoma, the tumor has a good prognosis [2, 8] with surgical resection alone (WHO grade 1).
Most previously reported cases of DIA were diagnosed in patients under 12 months of age [2, 7, 8, 9, 12, 13]. To the best of our knowledge, only three non-infantile cases of DIA have been reported [1, 6, 7]. We describe a rare non-infantile case in a 9-year-old boy and discuss its clinico-pathological aspects.
Case report
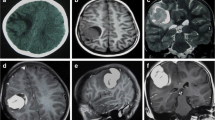
A 9-year-old boy was admitted to our hospital with a 2-day history of increasing motor weakness and sensory disturbance in his right upper and lower limbs. His birth and past development had been unremarkable. CT and MRI showed a large, contrast-enhanced cystic tumor in the left sensorimotor area (Fig. 1), which was shown as an avascular area on an angiogram. The tumor was partly shown as a hot area in both methionine and FDG PET imaging. We resected the entire tumor. Intraoperatively, the tumor was revealed to be attached to both the dura and the falx, and was clearly demarcated from the cerebral parenchyma, excluding partly the lateral side. Moreover, a mix of old and fresh hematomas was included in the tumor. Histopathological examination showed a highly cellular tumor with a marked desmoplastic component. However, mitosis and vascular proliferation were scant (Fig. 2). The tumor cells were positive for GFAP and vimentin but negative for neurofilament and class III β-tubulin in an immunohistochemical study. The MIB-1 labeling index was 1% or less. Ultrastructurally, the tumor cells contained large amounts of intermediate filaments occupying most of the cytoplasm. The tumor cells were surrounded by basement membrane (Fig. 3). Neuronal cells with dense core vesicles or microtubules were not identified. The histological diagnosis was DIA.
The patient’s neurological symptoms improved postoperatively. Neither radiotherapy nor chemotherapy was used postoperatively. He developed normally and had been doing well for 12 months after surgery without tumor recurrence.
Discussion
Six cases of DIA were reported by Taratuto et al. [13] in 1984 under the label of “superficial cerebral astrocytoma attached to the dura.” The tumors consisted of prominent desmoplastic stroma with a neuroepithelial population, mainly restricted to astrocytes that were immunopositive for GFAP and vimentin. On CT and MRI, the tumor is a large cystic mass with peripheral solid components that show prominent contrast enhancement. Desmoplastic infantile ganglioglioma (DIG), pleomorphic xanthoastrocytoma (PXA), meningioma, low-grade astrocytoma, glioblastoma, oligodendroglioma, and PNET are the objects of a differential diagnosis [3, 7, 8].
Histologically, DIA and DIG are almost identical except for the presence of neuronal cell differentiation in DIG. Presentation of both DIA and DIG usually occurs in the 1st year of life. VandenBerg et al. suggested that DIA was incomplete DIG with differentiation restricted to the astrocytic line [14]. The WHO tumor classification regards DIA as a subtype of DIG. PXA is considered to be a similar disease to DIG/DIA [8]. PXA is histologically characterized by intracellular lipid in addition to its desmoplastic features and may present much later, often in the second decade of life or even later [4], and often has similar clinicopathological features to DIA. De Chadarevian et al. suggested that DIA represents the infantile prelipidized form of PXA seen in older children and young adults [2]. Lellouch-Tubuiana et al. and other authors hypothesized that these tumors were derived from common origins with different histological differentiation and desmoplastic neuroepithelial tumor was proposed as the generic name for these tumors [7, 10]. PXA has a higher rate of recurrence after total removal than DIG/DIA. Therefore, if DIA is considered to be the infantile form of PXA, prudent postoperative observation is needed. In our case, we did not detect pleomorphism or lipidization of the tumor cells that would suggest differentiation to PXA.
Most previously reported cases of DIA were diagnosed in patients under 12 months of age. We have found only three non-infantile cases of DIA reported in the literature [1, 6, 7]. In the case reported by Kurose et al. [6], a cystic lesion of the right hemisphere was noticed when the patient was 6 months old, but it was not regarded as a tumor, and was not removed until the patient was 9 years old. This indicates that a favorable character of DIA is that it does not change for a long time. Moreover, in this case, glial cells with marked nuclear atypia and prominent pleomorphism were intermingled with cells typical of DIA. In the second case, a 7-year-old patient whose first symptom was a seizure at the age of 3 months [1], the pathological features were typical of DIA. In the third reported case, diagnosed in a 3-year-old patient [7], there was no detailed description of the pathological features. In our case, the pathological features were typical of DIA. Concerning DIG, two non-infantile cases arising in a 15-year-old patient and in a 25-year-old patient were reported [5]. This is the very rare reported case of DIA in which the patient was completely asymptomatic up to 9 years of age or more.
Electron microscopy revealed that tumor cells were surrounded by basal lamina in DIA; this does not exist in ordinary astrocytoma. Therefore, it has been postulated that DIA arises from subpial astrocytes that are known to produce basal lamina in the normal brain [8, 13]. Basal lamina matrix protein has been considered to inhibit growth and promote differentiation in glioma cells [8, 11]. The favorable prognosis of the tumor may be due to growth inhibition mediated by autocrine production of basal lamina components and/or extracellular matrix-induced maturation of undifferentiated cells.
If total surgical resection can be achieved, postoperative therapy such as radiotherapy and chemotherapy may not be required [7]. In our case, the patient developed normally and had been doing well for 12 months after surgery without tumor recurrence. Correct histological diagnosis of DIA is essential for determining postoperative treatment. The tumor accompanied by cysts in a child’s cerebral hemisphere should be considered as possibly being DIA.
References
Chacko G, Chandi SM, Chandy MJ (1995) Desmoplastic low grade astrocytoma: a case report and review of literature. Clin Neurol Neurosurg 97:32–35
De Chadarevian J-P, Pattisapu JV, Faerber EN (1990) Desmoplastic cerebral astrocytoma of infancy: light microscopy, immunohistochemistry, and ultrastructure. Cancer 66:173–179
Finizio FS (1995) CT and MRI aspects of supratentorial hemispheric tumors of childhood and adolescence. Childs Nerv Syst 11:559–567
Kepes JJ, Rubinstein LJ, Eng LF (1979) Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer 44:1839–1852
Kuchelmeister K, Bergmann M, von Wild K, Hochreuther D, Busch G, Gullotta F (1993) Desmoplastic ganglioglioma: report of two non-infantile cases. Acta Neuropathol (Berl) 85:199–204
Kurose A, Beppu T, Miura Y, Suzuki M, Ogawa A, Arai H, Kubo Y, Sugawara A, Sawai T (2000) Desmoplastic cerebral astrocytoma of infancy intermingling with atypical glial cells. Pathol Int 50:744–749
Lellouch-Tubuiana MC, Salazar C, Cinalli G, Renier D, Sainte-Rose C, Pierre-Kahn A, Zerah M (2000) The management of desmoplastic neuroepithelial tumors in childhood. Childs Nerv Syst 16:8–14
Louis DN, von Deimling A, Dickersin GR, Dooling EC, Seizinger BR (1992) Desmoplastic cerebral astrocytomas of infancy: a histopathologic, immunohistochemical, ultrastructural, and molecular genetic study. Hum Pathol 23:1402–1409
Park K, Yoo J, Cho H, Cho W, Park S (1998) Desmoplastic cerebral astrocytoma of infancy: a case report. J Korean Med Sci 13:440–444
Paulus W, Schlote W, Perentes E, Jacobi G, Warmuth-Metz M, Roggendorf W (1992) Desmoplastic supratentorial neuroepithelial tumours of infancy. Histopathology 21:43–49
Rutka JT, Giblin JR, Apodaca G, DeArmond SJ, Stern R, Rosenblum ML (1987) Inhibition of growth and induction of differentiation in a malignant human glioma cell line by normal leptomeningeal extracellular matrix proteins. Cancer Res 47:3515–3522
Sugiyama K, Arita K, Shima T, Nakaoka M, Matsuoka T, Taniguchi E, Okamura T, Yamasaki H, Kajiwara Y, Kurisu K (2002) Good clinical course in infants with desmoplastic cerebral neuroepithelial tumor treated by surgery alone. J Neurooncol 59:63–69
Taratuto AL, Monges J, Lylyk P, Leiguarda R (1984) Superficial cerebral astrocytoma attached to dura; report of six cases in infants. Cancer 54:2505–2512
VandenBerg SR, May EE, Rubinstein LJ, Herman MM, Perentes E, Vinores SA, Collins VP, Park TS (1987) Desmoplastic supratentorial neuroepithelial tumors of infancy with divergent differentiation potential (“desmoplastic infantile gangliogliomas”). Report on 11 cases of a distinctive embryonal tumor with favorable prognosis. J Neurosurg 66:58–71
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kato, M., Yano, H., Okumura, A. et al. A non-infantile case of desmoplastic infantile astrocytoma. Childs Nerv Syst 20, 499–501 (2004). https://doi.org/10.1007/s00381-003-0888-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-003-0888-9