Abstract
Case report
We report a case of a primary cranial chloroma in boy aged 2 years and 8 months. The symptoms were progressive bilateral exophthalmos, right abducens palsy, and bilateral papilledema. The tumor was partially calcified and was a round mass located in the bilateral sphenoidal bone extending into the orbit. Laboratory study did not show hematological abnormality. The tumor was partially removed by bilateral frontotemporal craniotomy and a diagnosis of primary granulocytic sarcoma was made from the surgical specimen. Progressive deterioration of visual acuity was seen and chemotherapy started on the 11th postoperative day followed by local cranium irradiation (24 Gy). The patient has been in complete remission for 37 months. The visual acuity recovered partially and follow-up magnetic resonance imaging showed a significant decrease in the size of the tumor.
Discussion
Radiological diagnosis of primary intracranial granulocytic sarcoma is difficult. Surgical removal may be an option for progressive neurological deterioration but chemotherapy is more important for both neurological stabilization and induction of remission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Granulocytic sarcoma is a solid tumor of immature granulocytes and usually occurs in patients with acute myelogenous leukemia (AML). It is also known as chloroma because of the greenish color made by the myeloperoxidase in the tumor cells when the specimen is fresh. Granulocytic sarcomas typically present in children with a mean age of about 7 to 8 years [16]. In the central nervous system, it is often found around the orbit in the course of AML [10, 13, 14]. The neurological signs vary relating to the mass effect of the tumor and the compression on the cranial nerves, resulting in proptosis, chemosis, exophthalmos, and cranial nerve deficits [5, 11, 16].
Granulocytic sarcomas that precede leukemia are called primary chloromas or primary granulocytic sarcomas. The preferred sites of these tumors are the small intestine, mediastinum, epidural site, uterus, and ovary. When laboratory studies do not suggest hematological disorders, preoperative diagnosis by radiological intervention is difficult. The tumors located intracranially may look like tumors of mesodermal origin [1, 4, 8, 17]. The role of surgery for primary cranial chloromas is controversial when the neurological deterioration due to mass effects is progressive. We present a case of a large primary chloroma in the sphenoidal bone and orbit of a boy aged 2 years and 8 months. Total removal was difficult due to intraoperative blood loss. Chemotherapy followed by local irradiation successfully completed remission. The deterioration in visual acuity was stabilized according to the remission. Follow-up magnetic resonance (MR) imaging showed a reduction in the mass after chemotherapy. The clinical course of this case is presented and primary cranial chloromas in the literature are reviewed.
Case report
A boy aged 2 years and 8 months was admitted to our hospital complaining of sporadic pain in both eyes in October 1999. Before admission, he was healthy, having a normal daily life, and had no past history of anemia or subcutaneous mass lesion. On admission, the physical examination was normal. No pathological enlargement of lymph nodes was noticed. The neurological examination revealed that he was alert and well orientated. Slight exophthalmos on both eyes were seen with right abducens palsy. Marked papilledema was noticed bilaterally. There was no congestion of the eyelids. Visual acuity as well as visual field was intact and the light reflex was prompt and complete. The laboratory data on admission were as follows: red blood cells 4,190,000/μl; white blood cells, 9,600/μl; Hb, 10.9 g/dl; Hct, 34.0%; MCV, 81.0; MCH, 26.0; MCHC, 32.1; Plt, 170,000/μl. Tumor makers AFP, CEA, and β2-microglobrin were all negative.
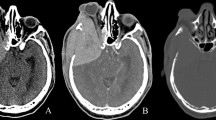
Computed tomography (CT) on admission demonstrated a large calcified tumor located in the sphenoidal bone and orbit (Fig. 1). It had a round shape with clear margins on both sides and the calcification was diffuse. Extension into the orbit was seen bilaterally. CT of the window level for bone density showed a slight osteolytic change in the right temporal bone and the lateral wall of the orbit. Enhancement was not significant in the tumor. T1-weighted MR imaging showed an isointense mass extending into both intraorbita and the middle fossa on both sides (Fig. 2). With Gd-enhancement, the tumor was enhanced homogeneously and involvement of the bilateral internal carotid arteries in the mass was seen. The coronal view on T1-weighted imaging showed a dural tail sign in the base of the middle fossa. T2-weighted MR imaging showed the tumor was homogeneously low-intense. Angiography showed no obvious staining of the tumor (Fig. 3). The internal carotid arteries were not occluded by the mass. The middle cerebral arteries were shifted upward by the tumor compression. Radio-isotope study using 99mTc-MDP showed an abnormal hot spot on the tumor. No other abnormal hot spots were seen (Fig. 3). Ultrasonography found no mass lesion in the abdomen. The extension of the tumor was significant on the optic nerve and the abducens nerve. Surgical decompression was scheduled with a preoperative diagnosis of fibrous dysplasia.
The axial views of magnetic resonance (MR) imaging show that the tumor was isointense on a T1-weighted image (WI; left upper) with diffuse Gd-enhancement (upper center). T2 WI showed a slightly low intensity (upper right). The coronal views (lower right on T1 WI with Gd enhancement and lower center on T2 WI) and the sagittal view (lower left on T1 WI with Gd enhancement) show that the tumor extended into both orbita and into the right-hand side of the middle fossa
The tumor was approached by bilateral frontotemporal craniotomy combined with a zygomatic approach on November 22nd. After a standard skin incision, a greenish brown-colored mass was seen under the right temporal muscle. The mass was soft and easily removed but associated with massive bleeding. The lateral half of the mass of the sphenoidal bone was removed on both sides but the orbital canal was not opened because the intraoperative bleed loss led to unstable systemic blood pressure. Blood transfusion was performed. With the greenish color of the tumor and intraoperative histological study in frozen tissue, the pathological diagnosis was chloroma.
The histological study of the tissue showed that the immature myeloid cells were medium-sized and looked like primitive cells (Fig. 4). Variation in cell morphology from round large nuclei to kidney-shaped nuclei was seen. Naphthol-ASD-cl staining was positive, indicating that the tumor cells were derived from granular cells. S-100 protein staining was negative. The histological diagnosis by permanent tissue was granulocytic sarcoma.
Papilledema as well as the abducens palsy disappeared after a few days postoperatively but the visual acuity deteriorated progressively. On the 6th postoperative day it was 20/100 in the left and 10/200 in the right. On the 9th postoperative day, the light reflex of both eyes became sluggish. On the following day, the pupils became dilated and the light reflexes disappeared. The laboratory study showed pancytopenia postoperatively and blood transfusion was performed again. Laboratory findings were red blood cells 2,950,000/μl; white blood cell, 7,900/μl; Hb, 8.8 g/dl; Hct, 26.3%; Plt, 135,000/μl. The differential counts of white blood cells were as follows: segmented neutrophils, 32%; lymphocytes, 36%; monocytes 7%; blasts, 16%. The bone marrow aspiration was obtained and showed relapsed AML of M3 type. On the 11th postoperative day, the patient underwent induction chemotherapy with 30 mg ATRA for 19 days, 110 mg cytarabine for 7 days, and 25 mg daunorubicin for 3 days. Cytogenic analysis then showed a +3, +6 inv(3) (p13 q27) but did not have t(15;17) translocation. Induction chemotherapy was changed to 80 mg etoposide for 5 days, 106 mg cytarabine for 7 days, and 2.7 mg mitoxantrone for 5 days. Bone marrow aspiration showed complete remission in February 2000. Consolidation chemotherapy was performed in the same month using 54 mg etoposide for 5 days, 1.62 g cytarabine for 3 days, and 5.4 mg idarubicin for 1 day. The chemotherapy continued with 80 mg etoposide for 3 days, 108 mg cytarabine for 5 days, and 2.6 mg mitoxantrone for 3 days in March 2000; 55 mg etoposide for 5 days, and 1.1 g cytarabine for 5 days in April 2000; 60 mg etoposide for 5 days, 1.7 g cytarabine for 3 days, and 5.4 mg idarubicin for 1 day in May 2000; and 90 mg etoposide for 3 days, 115 mg cytarabine for 5 days, and 3.0 mg mitoxantrone for 3 days in July 2000. Local radiation at 24 Gy was performed in November 2000. In the mean time, intrathecal injection of methotrexate, cytarabine, and hydrocortisone was performed four times. Bone marrow transplantation was not performed.
After the induction of chemotherapy, the visual acuity deteriorated progressively for 4 months, after which it was 20/100 in the left eye and finger counting in the right. The tumor did not increase in size, as shown by MR imaging (Fig. 5). From April 2000, the visual acuity showed improvement and was 20/100 in the left eye and 10/200 in the right in November 2000. In December 2002, the latest MR imaging showed a significant decrease in the size of the tumor after the chemotherapy followed by irradiation (Fig. 5). The visual acuity was between 6/200 and 10/200 in the right eye and 8/200 in the left. No other neurological deficits were seen. The patient has been in complete remission for 39 months. His mental development is normal and he is attending a regular school, but the height and the body weight are less than −2SD of the average.
MR imaging performed after surgery and before chemotherapy on T1 WI (upper left), T2 WI (upper center), and T1 WI with Gd enhancement (upper right) shows residual tumor in the orbit, in the sphenoidal sinus, and around the bilateral internal carotid arteries. MR imaging 3 years after the surgery on T1 WI (lower left), T2 WI (lower center), and T1 WI with Gd enhancement (lower right) shows a significant decrease in the size of the tumor
Discussion
Granulocytic sarcomas are rare extramedullary tumors of myeloid origin and are often found in the presence of AML. The median survival was reported to be 22 months [16]. In the course of AML, additional occurrence of granulocytic sarcoma despite antileukemic treatment is a sign of relapse and indicates poor prognosis. Surgical removal and irradiation may control the local growth of tumor but does not influence survival [7].
Primary granulocytic sarcoma, in which tumors precede the development of leukemia, tend to progress to leukemia [2, 12]. Our case had no hematological abnormality before the surgery but within 10 days of surgery, it became overt. Although the incidence of developing leukemia is not well known, early antileukemic chemotherapy followed by intensified consolidation are feasible. In the literature published between 1992 and 2002, 8 cases of primary granulocytic sarcoma of cranial lesion have been reported (Table 1) [2, 3, 5, 6, 15, 16, 18]. The histological diagnosis was confirmed by biopsy in most of the cases. Craniotomy was performed in two cases. The reasons for craniotomy were the difficult location of the tumor for biopsy (skull base and brain parenchyma) and the rapid progress of the neurological deterioration. None of the patients treated surgically underwent total removal of the tumor. All of the reported cases had neurological deterioration due to the tumor compression but neurological signs disappeared or at least became stabilized by postoperative antileukemic therapy. Follow-up radiological imaging also showed the disappearance or a reduction in the size of the tumor. None of the 9 cases, including our case, resulted in death. Seven patients achieved complete remission with an average follow-up period of 36.1 months (range 13–72 months). Five patients received local irradiation combined with chemotherapy.
A literature review demonstrated that the nonleukemic period after the diagnosis of primary granulocytic sarcoma was significantly longer in patients who had systemic chemotherapy than in others treated only by surgery or local radiation [19]. Primary cranial granulocytic sarcoma is rare and its radiographical features are not characteristic [10, 13, 14]. The histological diagnosis is the key and it is recommended to perform biopsy first. Not only the histological diagnosis but also the cytogenetic analysis to detect specific chromosomal abnormalities can be made from tissue obtained by biopsy in order to decide on the appropriate chemotherapy protocol [9].
The critical factor for a good outcome in primary granulocytic sarcoma is not the management of mass but how immediately the antileukemic chemotherapy can be induced while patients are in the nonleukemia stage. In our case, the diagnosis was made by the tissue obtained by craniotomy but the patient showed hematological abnormality soon after the surgery. The induction of chemotherapy could have been done in our case while the patient was still nonleukemic if we had performed a less invasive biopsy before the craniotomy. Although the management of intracranial lesions in advanced stages of AML is likely to be difficult, granulocytic sarcoma as a primary disease in the cranium should be considered differently. Our case as well as the literature demonstrated the effectiveness of chemotherapy at controlling the tumor mass even with progressive neurological deterioration. Surgical treatment may be one of the options when making a diagnosis. Aggressive and invasive surgical removal is not necessary and early induction of appropriate chemotherapy is important.
References
Ahn JY, Choi EW, Kang SH, Kim YR (2002) Isolated meningeal chloroma (granulocytic sarcoma) in a child with acute lymphoblastic leukemia mimicking a falx meningioma. Childs Nerv Syst 18:153–156
Barnett MJ, Zussman WV (1986) Granulocytic sarcoma of the brain: a case report and review of the literature. Radiology 160:223–225
Binder C, Tiemann M, Haase D, Humpe A, Kneba M (2000) Isolated meningeal chloroma (granulocytic sarcoma)—a case report and review of the literature. Ann Hematol 79:459–462
Bulas RB, Laine FJ, Das Narla L (1995) Bilateral orbital granulocytic sarcoma (chloroma) preceding the blast phase of acute myelogenous leukemia: CT findings. Pediatr Radiol 25:488–489
Chen CY, Zimmerman RA, Faro S, Bilaniuk LT, Chou TY, Molloy PT (1996) Childhood leukemia: central nervous system abnormalities during and after treatment. Am J Neuroradiol 17:295–310
Deme S, Deodhare SS, Tucker WS, Bilbao JM (1997) Granulocytic sarcoma of the spine in nonleukemic patients: report of three cases. Neurosurgery 40:1283–1287
Fiorillo A, de Rosa G, Canale G, Fariello I, D'Amore R, Bonavolonta G (1996) Granulocytic sarcoma in nonleukemic children: report of two new cases successfully treated by local radiation therapy and systemic chemotherapy. Haematologica 81:155–158
Fruauff AA, Barasch ES, Rosenthal A (1988) Solitary myeloblastoma presenting as acute hydrocephalus: review of literature, implications for therapy. Pediatr Radiol 18:369–372
Girardot PM, Mathiot C, Validire P, Klijanienko J, Saidi A, Bessa E, Quintana E, Vielh P (1996) Cerebrospinal fluid examination in a case of childhood orbital granulocytic sarcoma. Diagn Cytopathol 15:237–240
Kao SC, Yuh WT, Sato Y, Barloon TJ (1987) Intracranial granulocytic sarcoma (chloroma): MR findings. J Comput Assist Tomogr 11:938–941
Lee B, Fatterpekar GM, Kim W, Som PM (2002) Granulocytic sarcoma of the temporal bone. Am J Neuroradiol 23:1497–1499
Levy R, Shvero J, Sandbank J (1989) Granulocytic sarcoma (chloroma) of the temporal bone. Int J Pediatr Otorhinolaryngol 18:163–169
Parker K, Hardjasudarma M, McClellan RL, Fowler MR, Milner JW (1996) MR features of an intracerebellar chloroma. Am J Neuroradiol 17:1592–1594
Pui MH, Fletcher BD, Langston JW (1994) Granulocytic sarcoma in childhood leukemia: imaging features. Radiology 190:698–702
Romaniuk CS (1992) Case report: granulocytic sarcoma (chloroma) presenting as a cerebellopontine angle mass. Clin Radiol 45:284–285
Stockl FA, Dolmetsch AM, Saornil MA, Font RL, Burnier MN Jr (1997) Orbital granulocytic sarcoma. Br J Ophthalmol 81:1084–1088
Velasco F, Ondarza R, Quiroz F, Arista J (1993) Meningioma-like intracranial granulocytic sarcoma (chloroma). Radiologic and surgical findings. Rev Invest Clin 45:473–478
Voessing R, Berthold F, Richard KE, Thun F, Schroeder R, Krueger GR (1992) Primary myeloblastoma of the pineal region. Clin Neuropathol 11:11–15
Wang AM, Lin JC, Power TC, Haykal HA, Zamani AA (1987) Chloroma of cerebellum, tentorium and occipital bone in acute myelogenous leukemia. Neuroradiology 29:590
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohta, K., Kondoh, T., Yasuo, K. et al. Primary granulocytic sarcoma in the sphenoidal bone and orbit. Childs Nerv Syst 19, 674–679 (2003). https://doi.org/10.1007/s00381-003-0797-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-003-0797-y