Abstract
Calcified lesion is a risk factor for adverse events, even in the drug-eluting stent (DES) era. Recently, drug-coated balloon (DCB) has been shown to have favourable results for in-stent restenosis and small vessels, but its results for calcified lesions are unknown. This study aimed to clarify the rotational atherectomy (RA) and DCB results for calcified lesions of nonsmall vessels. A total of 194 consecutive de novo lesions from 165 cases underwent RA for calcified lesions of nonsmall vessels between January 2016 and August 2018 in a single centre. Overall, 8 cases/10 lesions were excluded because of RA followed plain old balloon angioplasty (POBA). Remaining lesions were grouped into the DES (88 cases/104 lesions) and DCB (69 cases/80 lesions) groups and then compared retrospectively. The primary endpoint was post-discharge major adverse cardiovascular events (MACE) at 1 year, and it was defined as cardiac death, noncardiac death, target-vessel-related myocardial infarction, target lesion revascularization (TLR), and major bleeding (BARC ≥ type 3). There was no difference in the clinical follow-up rate between RA + DES (96/104 lesions) and RA + DCB (78/80 lesions). The post-discharge MACE values after 1 year of RA + DES and RA + DCB were 8% and 11% (P = 0.30), respectively, in terms of cardiac death (0% vs. 0%, respectively), noncardiac death (4% vs. 3%, respectively, P = 0.36), target-vessel-related myocardial infarction (0% vs. 0%, respectively), TLR (4% vs. 8%, respectively, P = 0.30), and major bleeding (1% vs. 0%, respectively). For calcified lesions of nonsmall vessels, RA + DCB showed good results as well as RA + DES. RA + DCB is a potential new strategy for these lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug-eluting stents (DES) have improved the clinical and procedural outcomes of patients with coronary artery disease. At present, most percutaneous coronary intervention (PCI) procedures have been accomplished with the use of DES. However, a calcified lesion is a risk factor for adverse events, even in the era of DES [1]. In fact, the rates of restenosis and stent thrombosis were higher for calcified lesions than for noncalcified lesions [2]. Rotational atherectomy (RA) beneficial for severely calcified lesions, because it allows smooth delivery of the stent to the target lesions and has less incidence of stent under expansion, which is a risk for stent thrombosis [3, 4]. PCI without stent placement is expected to eliminate the disadvantage of longer dual antiplatelet therapy (DAPT) with DES placement. Drug-coated balloon (DCB) is a new therapeutic coronary intervention that has been effective for in-stent restenosis, bifurcated lesions, and small vessels [5,6,7,8,9]. Recently, the use of DCB was associated with lower mortality when compared with control treatments [10]. However, a severely calcified lesion is a risk factor for DCB restenosis [11]. To address this issue, the new intervention strategy has been the reduction of the calcium volume using RA, followed by DCB. In the present study, we aimed to compare the clinical outcomes between DES and DCB among patients undergoing RA for calcified lesions of nonsmall vessels.
Methods
Study design
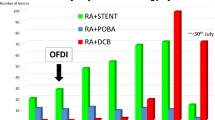
A total of 1991 lesions (1441 cases) underwent PCI between January 2016 and August 2018 at Kyoto Katsura Hospital, Cardiovascular Center. Of these, 472 lesions (24%) were treated with RA. We retrospectively analysed 194 consecutive lesions of 165 cases that underwent RA for de novo calcified lesions of nonsmall vessels, which were defined as those having a reference vessel diameter (RVD) of ≥ 2.5 mm by quantitative coronary angiography (QCA). RA was followed by DES placement in 55%, DCB placement in 41%, and POBA in 4%. Patients were grouped into the RA + DES (88 cases/104 lesions) or RA + DCB (69 cases/80 lesions) group. Cases that underwent RA + POBA were excluded from the analysis because of the small number (Fig. 1a). The transition of our treatment strategy is described on the bar graph (Fig. 1b). RA + DES was the main treatment before 2016. If stent placement was to be avoided for several reasons, RA + POBA was performed instead. However, in this series, RA + POBA had a high restenosis rate of 70% (7/10 lesions). When the effectiveness of DCB was proven, cases of RA + DCB gradually increased from 2016 and exceeded the number of RA + DES cases in 2018. We compared the acute and 1-year clinical outcomes between the groups.
a Flowchart of study population inclusion. The study population was identified from a percutaneous coronary intervention database between January 2016 and April 2018. b Trend of adjunctive strategy for Rota in nonsmall calcified lesions. PCI = percutaneous coronary intervention; Rota: rotational atherectomy; DES: drug-eluting stent; DCB: drug-coated balloon
PCI was performed after obtaining written informed consent from each patient. Patients with symptomatically documented coronary artery disease with moderate or severely calcified de novo stenosis of > 50% were enrolled. The coronary lesion type was categorized based on the American Heart Association/American College of Cardiology classification. The severity of calcification was classified as (1) severe, when a density was noted without cardiac motion before contrast injection and when both sides of the arterial wall were generally involved; (2) moderate, when the density was noted only during the cardiac cycle before contrast injection; (3) mild, for lesions other than severe and moderate; or (4) absent.
Six types of DES (Xience; Abbott Vascular, Abbott Park, IL, USA, Synergy; Boston Scientific, Natick, MA, USA, Nobori; Ultimaster; Terumo Corporation, Shibuya-ku, Tokyo, Japan, Resolute Onyx; Medtronic, CA, US and Biofreedom; Biosensors Interventional Technologies, Singapore) were used based on the operator’s judgement, and one DCB (Sequent Please; B Braun, Melsungen, Germany) was used. The PCIs were performed with image guidance using optical frequency domain imaging (OFDI; Lunawave; Terumo Corporation, Tokyo, Japan) or intravascular ultrasound (IVUS; Atlantis; Boston Scientific Corporation, Natick, MA, USA). RA was performed using the Rotablator (Boston Scientific, Natic, MA).
Medications
Patients received dual antiplatelet therapy (DAPT) with oral aspirin (100 mg) and clopidogrel (75 mg) or prasugrel (3.75 mg). In case of emergent PCI, aspirin (300 mg) and prasugrel (20 mg) were administered before PCI. Heparin was administered to maintain an activated clotting time of > 300 s during the procedures. The minimum period for DAPT use was 10 months for DES and 3 months for DCB.
Quantitative coronary angiography analysis
Using QAngio XA version7.3 (MEDIS Medical Imaging System BV, Leiden, The Netherlands), QCA was performed at baseline, at the end of the PCI, and on follow-up after intracoronary nitrate injection. The minimum lumen diameter (MLD), reference diameter (RVD), and % diameter stenosis (DS) were measured. Acute gain was defined as an increase in MLD after intervention. Late lumen loss (LLL) was defined as a decrease in MLD on follow-up, compared with that after intervention. Restenosis was defined as %DS of ≥ 50%. Late lumen enlargement (LLE) was defined as negative LLL.
This study was approved by the Ethics Committee (IRB) of The Kyoto Katsura Hospital Ethic and Clinical Research Committee (IRB No: 693 and IRB approval date, March 24, 2020).
Endpoint and definitions
Angiographic success was defined as a minimum DS of < 30%, as visually assessed on angiography, and a thrombolysis in myocardial infarction grade of III at the end of the procedure. Procedural success was defined as angiographic success without in-hospital complications of cardiac death, noncardiac death, QMI, NQMI, TLR, and emergency coronary artery bypass graft surgery. MI was defined as elevation of CK-MB to greater than 10 times the upper limit of normal [12]. QMI was defined as development of a new Q wave (> 0.4 s) in the electrocardiogram.
The primary endpoint was major adverse cardiovascular events (MACE) at 1 year. MACE was defined as cardiac death, noncardiac death, target-vessel-related myocardial infarction or target lesion revascularization (TLR), and major bleeding (Bleeding Academic Research Consortium (BARC) ≥ type 3) [13]. Follow-up angiography was scheduled at 6–8 months.
Statistical analysis
Statistical analyses of the recorded data were performed using the Excel statistical software package (Ekuseru-Toukei 2015; Social Survey Research Information Co., Ltd., Tokyo, Japan). Continuous variables were described as mean ± SD and were compared using t test. Categorical variables were described by the absolute counts and were compared using Pearson Chi square or Fisher’s exact test. P values of < 0.05 were considered significant. Kaplan–Meier curves were used to visualize the time to events after discharge. Survival estimates were compared using the log-rank test.
Results
Representative cases
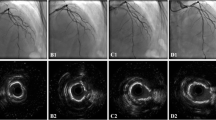
Case 1 underwent RA + DES (Fig. 2). The patient was an 80-year-old woman who was on haemodialysis and had risk factors of hypertension, diabetes, and diagnosed stable angina. Severe calcification was located in the mid left anterior descending (LAD) artery. OFDI showed a calcified plaque from the 12 o’clock to the 7 o’clock positions. The lesion was treated with RA (burr size of 2.0 mm), which increased the lumen area from 1.45 to 2.8 mm2. After the use of a cutting balloon plus DES, the final lumen area was 5.60 mm2. Follow-up coronary angiography showed no restenosis. QCA showed a 0.03-mm LLL.
Case 2 underwent RA + DCB (Fig. 3). The patient was a 65-year-old man who had had hypertension, chronic kidney disease (estimated glomerular filtration rate = 45 mL/mm/1.73 m2), and diagnosed stable angina. Severe calcification was located in the mid LAD. OFDI showed a calcified plaque from the 3 o’clock to the 6 o’clock positions. This severely calcified lesion was treated with RA (burr size: 2.25 mm), which increased the lumen area from 2.07 to 3.57 mm2. After the use of a cutting balloon plus DCB, the final lumen area was 5.20 mm2. Follow-up coronary angiography at 6 months showed no restenosis. QCA data revealed a negative LLL of 0.10 mm.
Patient and angiographic characteristics
The patient and angiographic characteristics are shown in Table 1. Compared with the RA + DES group, the RA + DCB group had frequent atrial fibrillation (28% vs. 6%, P = 0.0090) but had more cases of multivessel disease (50% vs. 38%, P = 0.012). The other factors were similar between the groups. There were no significant differences in the angiographic characteristics between the groups at baseline.
Procedural data
The procedural data are shown in Table 2. Compared with the RA + DES group, the RA + DCB group had a higher rotational speed (155,000 ± 10,000 rpm vs. 160,000 ± 130,000 rpm, P = 0.0018, similar burr/artery ratio 0.64 ± 0.10 vs. 0.67 ± 0.11, respectively, P = 0.080), and higher predilatation balloon pressure (13 ± 3.9 atm vs. 11 ± 2.0 atm, P = 0.016). The rate of dissection after predilatation was not different between the groups. There was no dissection in the RA + DES group at the end. In the RA + DCB group, dissection (NHLBI classification) at the end was found in 15 lesions for type A, 10 lesions for type B, and 8 lesions for type C, and there was no type D or higher dissection.
In-hospital results
The procedural results are shown in Table 3. The RA + DES and RA + DCB groups had no differences in angiographic success rate (98% vs. 96%, respectively) and procedural success (98% vs. 94%, respectively). There was one case of NQMI with a maximum CK-MB of 151 mg/dL in the RA + DCB group.
MACE at 1 year
The post-discharge MACE at 1 year is shown in Table 4. Clinical follow-up was similar performed in 95% (84/88) of the RA + DES group and in 98% (68/69) of the RA + DCB group. There was no difference in post-discharge MACE between the groups (8% vs. 11%, P = 0.30). There was one case on DAPT who developed cerebral haemorrhage at 9 months in the RA + DES group. Patients who were on DAPT for more than 1 year were predominant in the RA + DES group. There was no difference in the Kaplan–Meier curve for post-discharge MACE between the two groups (Fig. 4).
QCA results
The QCA results are shown in Table 5. Compared with the RA + DCB group, the RA + DES group had similar preprocedural RVD (3.03 ± 0.36 vs. 2.97 ± 0.45 mm, P = 0.40) but significantly larger postprocedural RVD (3.39 ± 0.51 vs. 3.07 ± 0.48 mm, P < 0.0001), post MLD (3.01 ± 0.26 vs. 2.39 ± 0.56 mm, P < 0.0001), and acute gain (1.71 ± 0.62 vs. 1.04 ± 0.61 mm, P < 0.0001). Follow-up CAG was performed in 70% (72/104) of the RA + DES group and in 73% (58/80) of the RA + DCB group (P = 0.69). On follow-up, the RA + DES group showed large RVD, MLD and lower DS.
However, LLE was also similar between the groups (0.29 ± 0.60 vs. 0.31 ± 0.67 mm, P = 0.83). The binary restenosis rate was similar between the RA + DES and RA + DCB groups (6% vs. 9%, respectively, P = 0.13).
Discussion
The Rotablator is an effective device for calcified lesions, but its ability to reduce calcium plaques can sometimes be difficult to judge. Moreover, slow flow during the use of the Rotablator in some cases is difficult to expect before the procedure. After the introduction of OFDI, with this, we can understand how the Rotablator works on calcified lesions more clearly than with IVUS [14]. Our RA strategy had been changed from modification to aggressive ablation. Previous studies on DES following the RA have shown a TLR rate of 6.8 to 11.7% [15,16,17,18]. Our data for the RA + DES group showed a TLR of 4%, which is lower than the previously reported rate. On the other hand, the TLR of our RA + DCB group was 8%, which was similar to the previous RA + DES results. RA + DCB results reported in 2017 by Rissanen showed an extremely low TLR rate (1.5% at 12 months) [19]. Subsequently, some studies in Japan also showed a TLR of 6–16%, which was similar to this data [14, 20, 21]. In Japan, high TLR rate was observed using follow-up CAG.
Few studies have reported the use of DCB for nonsmall vessels. It has been reported that with DCB treatment, the clinical outcomes in the calcified group were similar to those in the noncalcified group when treated with DCB [22]. We were confident to use DCB after effective calcium plaque reduction by RA.
The advantage of RA + DCB over RA + DES was the shorter DAPT and the fact that stent does not leave in the body. Basically, most patients with severely calcified lesions have high bleeding and thrombotic risks. Adapting the Credo Kyoto bleeding and thrombotic risk score [23] for this study population, 56% can be categorized to high bleeding risk, and 60% can be categorized to high thrombotic risk. Elderly patients who had relatively more chance of developing triple therapy-requiring atrial fibrillation after stenting can be categorized to the high bleeding risk group [24]. Bleeding after PCI can increase the risk for death [25]. In this context, DCB had a low event risk in the DEBUT trial [11]. In our study, the RA + DCB group showed relatively higher restenosis rate, but it did not have any risk for stent thrombosis. Although stent thrombosis has been rare recently, its mortality rate is very high once it occurs [26]. In addition, when the stent is not well expanded because of insufficient lesion preparation, the risk for stent thrombosis increases [27]. Another issue would be the possibility of DES inducing chronic vessel wall inflammation because of the existence of the polymer and the delayed intimal healing, which can lead to neoatherosclerosis [28,29,30]. Neoatherosclerosis has been reported to be associated with subacute stent thrombosis, late stent thrombosis, and very late stent thrombosis. Therefore, patients in whom DES is placed are more likely to continue DAPT for 1 year to prevent stent thrombosis [31]. The presence of very large residual calcification after RA increases the risk for stent under expansion. DCB is an alternative to stenting in cases of inadequate lesion preparation.
In this study, the RA + DCB group showed only low-grade dissection. In cases with type D or worse dissection, a bailout stent would have been required. In one report, the type A to C dissections that occurred in almost all lesions treated with DCB were shown to have healed on follow-up angiography [32]. Patients who underwent follow-up angiography showed that the dissection was healed, and there was no target-vessel-related myocardial infarction, even though leaving dissections.
The rate of LLE was lower in this present study (20%) than in the previous report of Funatsu et al., who reported LLE in 56% of cases treated with DCB for lesions of small vessel disease [33]. In calcified lesions, LLE may not occur easily. It has been reported that LLE is likely to occur when dissection occurs, but the rate of dissection was low in this study [33].
The relatively high number of patients with multivessel disease in the RA + DES group in this study may have been because of our strategy on the selection of the secure PCI modality based on patient background.
To complete DCB use, it is necessary to acquire a larger lumen area and avoid recoil and dissection. Therefore, we determined calcification by control angiogram and selected OFDI or IVUS to check for wire bias. Imaging modality was used in 100% of patients, and 82% underwent OFDI. When the wire touched the surface of the calcified plaque, we applied RA to reduce the calcified plaque. This may have led to the relatively large burr/artery ratio. Moreover, we clearly understand the possible bias on residual calcium thickness and wire. A burr/artery ratio of > 0.6 was reported to be an indication for a less need for revascularization [34]. A burr/artery ratio of > 0.6 might have led to the low restenosis rate in both groups of this study. In more than half of the cases in both groups (RA + DES group 53% and RA + DCB group 58%), more than one burr was used. These data supported the aggressive debulking strategy to reduce the incidence of MACE.
Limitations
This study had several limitations. First was the single-centre, nonrandomized, retrospective design with a relatively small sample size and the absence of a full angiographic follow-up. Second, the details of the procedures, such as the use of stent and standard or scoring balloon, were based on the operator’s judgement. Furthermore, although DAPT was administered in all cases, its duration was at the discretion of each doctor. Our strategy has gradually changed from DES to DCB, considering that the debulking effect can be assessed by imaging modality.
Conclusion
For calcified lesions of nonsmall vessels, RA + DCB showed good results as well as RA + DES. RA + DCB is a potential new strategy for these lesions.
References
Huang BT, Huang FY, Zuo ZL, Liu W, Huang KS, Liao YB, Wang PJ, Peng Y, Zhang C, Zhao ZG, Huang DJ, Chen M (2015) Target lesion calcification and risk of adverse outcomes in patients with drug-eluting stents. A meta-analysis. Herz 40:1097–1106
Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P (2014) Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 63:1703–1714
Cortese B, Aranzulla TC, Godino C, Chizzola G, Zavalloni D, Tavasci E, De Benedictis M, Ettori F, Presbitero P, Colombo A (2016) Drug-eluting stent use after coronary atherectomy: results from a multicentre experience—The ROTALINK I study. J Cardiovasc Med 17:665–672
Jinnouchi H, Kuramitsu S, Shinozaki T, Hiromasa T, Kobayashi Y, Takeji Y, Miura M, Masuda H, Matsumura Y, Yamaji Y, Sakakura K (2018) Five-year clinical outcomes after drug-eluting stent implantation following rotational atherectomy for heavily calcified lesions. Circ J 82:983–991
Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Matthias Schreiber M, Mahfoud F, Linke A, Stephan FP, Mueller C, Rickenbacher P, Coslovsky M, Gilgen N, Osswald S, Kaiser C, Scheller B (2018) Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2). Lancet 392:849–856
Rissanen TT, Uskela S, Eränen J, Mäntylä P, Olli A, Romppanen H, Antti Siljander A, Pietilä M, Minkkinen MJ, Tervo J, Kärkkäinen JM (2019) Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trials. Lancet 394:230–239
Buccheri D, Lombardo RM, Cortese B (2019) Drug-coated balloons for coronary artery disease: current concepts and controversies. Future Cardiol 15:437–454
Cortese B, Piraino D, Buccheri D, Alfonso F (2016) Treatment of bifurcation lesions with drug-coated balloons: a review of currently available scientific data. Int J Cardiol 220:589–594
Jeger RV, Eccleshall S, Ahmad WAW, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FX (2020) Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv 13:1391–1402
Scheller B, Vukadinovic D, Jeger R, Rissanen TT, Scholz SS, Byrne R, Kleber FX, Latib A, Clever YP, Ewen S, Böhm M, Yang Y, Lansky A, Mahfoud F (2020) Survival after coronary revascularization with paclitaxel-coated balloons. J Am Coll Cardiol 75:1017–1028
Cortese B, D’Ascenzo F, Fetiveau R, Balian V, Blengino S, Fineschi M, Rogacka R, Lettieri C, Pavei A, D’Amico M, Poli A (2018) Treatment of coronary artery disease with a new-generation drug-coated balloon: final results of the Italian Elutax SV rEgistry-DCB-RISE. J Cardiovasc Med (Hagerstown) 19:247–252
Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW (2013) Consideration of a new definition of clinically relevant myocardial infarction after coronary revasculrarization. An expert consensus document from the Society for Cardiovascular Angiography and Intervention (SCAI). J Am Coll Cardiol 62:1563–1570
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123:2736–2747
Nagai T, Mizobuchi M, Funatsu A, Kobayashi T, Nakamura S (2019) Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. https://doi.org/10.1007/s12928-019-00611-y
Abdel-Wahab M, Baev R, Dieker P, Kassner G, Khattab AA, Toelg R, Sulimov D, Geist V, Richardt G (2013) Long-term clinical outcome of rotational atherectomy followed by drug-eluting stent implantation in complex calcified coronary lesions. Catheter Cardiovasc Interv 81:285–291
Furuichi S, Sangiorgi GM, Godino C, Airoldi F, Montorfano M, Chieffo A, Michev I, Carlino M, Colombo A (2009) Rotational atherectomy followed by drug-eluting stent implantation in calcified coronary lesions. EuroIntervention 5:370–374
Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, Schofer J, King L, Neumann FJ, Khattab AA (2013) High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv 6:10–19
Tian W, Mahmoudi M, Lhermusier T, Pendyala LK, Kiramijyan S, Saar M, Ota H, Chen F, Torguson R, Suddath WO, Satler LF (2015) Clinical outcomes of first- and second-generation drug-eluting stents in patients undergoing rotational atherectomy for heavily calcified coronary lesions. Cardiovasc Revasc Med 16:147–150
Rissanen TT, Uskela S, Siljander A, Kärkkäinen JM, Mäntylä P, Eränen J (2017) Percutaneous coronary intervention of complex calcified lesions with drug-coated balloon after rotational atherectomy. J Interv Cardiol 30:139–146
Shiraishi J, Kataoka E, Ozawa T, Shiraga A, Ikemura N, Matsubara Y, Nishimura T, Ito D, Kojima A, Kimura M, Kishita E, Nakagawa Y, Hyogo M, Sawada T (2019) Angiographic and clinical outcomes after stent-less coronary intervention using rotational atherectomy and drug-coated balloon in patients with de novo lesions. Cardiovasc Revasc MED 21:647–653
Ueno K, Morita N, Kojima Y, Takahashi H, Kawasaki M, Ito R, Kondo H, Motoguchi S, Yoshida T, Hashimoto Y, Tatsumi T, Kitamura T (2019) Safety and long-term efficacy of drug-coated balloon angioplasty following rotational atherectomy for severely calcified coronary lesions compared with new generation drug-eluting stents. J Interv Cardiol 2019:9094178
Ito R, Ueno K, Yoshida T, Takahashi H, Tatsumi T, Hashimoto Y, Kojima Y, Kitamura T, Morita N (2018) Outcomes after drug-coated balloon treatment for patients with calcified coronary lesions. J Interv Cardiol 31:436–441
Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Akasaka T (2018) Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc 7:e008708
Faza NN, Mentias A, Parashar A, Chaudhury P, Barakat AF, Agarwal S, Wayangankar S, Ellis SG, Murat Tuzcu E, Kapadia SR (2017) Bleeding complications of triple antithrombotic therapy after percutaneous coronary interventions. Catheter Cardiovasc Interv 89:E64–E74
Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL (2015) Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol 66:1036–1045
Holmes DR Jr, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, Williams DO, Kimura T, Moliterno DJ (2010) Stent thrombosis. J Am Coll Cardiol 56:1357–1365
Cheneau E, Leborgne L, Mintz GS, Kotani JI, Pichard AD, Satler LF, Canos D, Castagna M, Weissman NJ, Waksman R (2003) Predictors of subacute stent thrombosis:results of a systematic intravascular ultrasound study. Circulation 108:43–47
Cui Y, Liu Y, Zhao F, Shi D, Chen K (2016) Neoatherosclerosis after drug-eluting stent implantation: roles and mechanisms. Oxid Med Cell Longev 2016:5924234
Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M (2015) Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 36:2147–2159
Nakamura D, Attizzani GF, Toma C, Sheth T, Wang W, Soud M, Aoun R, Tummala R, Leygerman M, Fares A, Mehanna E (2016) Failure mechanisms and neoatherosclerosis patterns in very late drug-eluting and bare-metal stent thrombosis. Circ Cardiovasc Interv 9:e003785
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr (2014) Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371:2155–2166
Cortese B, Silva Orrego P, Agostoni P, Buccheri D, Piraino D, Andolina G, Seregni RG (2015) Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv 8:2003–2009
Funatsu A, Nakamura S, Inoue N, Nanto S, Nakamura M, Iwabuchi M, Ando K, Asano R, Habara S, Saito S, Mitsudo K (2017) A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin Res Cardiol 106:824–832
Kaplan BM, Safian RD, Mojares JJ, Reddy VM, Gangadharan V, Schreiber TL, Grines CL, O’Neill WW (1996) Optimal burr and adjunctive balloon sizing reduces the need for target artery revascularization after coronary mechanical rotational atherectomy. Am J Cardiol 78:1224–1229
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iwasaki, Y., Koike, J., Ko, T. et al. Comparison of drug-eluting stents vs. drug-coated balloon after rotational atherectomy for severely calcified lesions of nonsmall vessels. Heart Vessels 36, 189–199 (2021). https://doi.org/10.1007/s00380-020-01684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01684-z