Abstract
HAS-BLED score was developed for bleeding prediction in patients with atrial fibrillation (AF). Recently, it was also used in patients undergoing percutaneous coronary interventions (PCI). This study analyzes the HAS-BLED predictivity for bleedings and mortality in patients with acute coronary syndromes (ACS) without AF, and evaluates the utilization of alternative criteria for renal dysfunction. The study population was composed of 704 patients with ACS. Six-hundred and eleven patients completed the follow-up. The HAS-BLED score was calculated both using the original definition of renal dysfunction, both using three alternative eGFR thresholds (< 30, < 60 and ≤ 90 ml/min/1.73 mq). In-hospital and post-discharge bleedings and mortality were recorded, and calibration and discrimination of the various risk models were evaluated using the Hosmer–Lemeshow test and the C-statistic. In-hospital bleedings were 4.7% and mortality was 2.7%. Post-discharge bleedings were 3.1% and mortality was 4.4%. Regarding bleeding events and in-hospital mortality, the HAS-BLED original risk model demonstrated a moderate-to-good discriminative performance (C-statistics from 0.65 to 0.76). No significant differences were found in predictive accuracy when applying alternative definitions of renal dysfunction based on eGFR, with the exception of post-discharge mortality, for which HAS-BLED model assuming an eGFR value < 60 ml/min/1.73 mq showed a discriminative performance significantly higher in comparison to the other risk models (C-statistic 0.71 versus 0.64–0.66). In conclusion, in our ACS population, the HAS-BLED risk score showed a fairly good predictive accuracy regarding in-hospital and follow-up bleeding events and in-hospital mortality. The use of renal dysfunction alternative criteria based on eGFR values resulted in out-of hospital mortality predictive accuracy enhancement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antithrombotic therapy represents the mainstay pharmacologic strategy both for patients affected by atrial fibrillation (AF) and for those with acute coronary syndromes (ACS). In the last decades, the use of new potent antithrombotic agents, the utilization of combined drugs regimens, the widespread application of invasive procedures, and the aging of the patients, all have determined an increase of bleeding events incidence. In particular, they represent the most common non-cardiac in-hospital complication in patients with ACS and they keep being a frequent event also during chronic maintenance phase [1,2,3,4,5]. Moreover, evidences exist about the worse clinical outcome conferred by bleeding in these patients, as it represents an independent risk factor for mortality, showing an hazard equivalent or greater than that of myocardial infarction [1, 6,7,8,9,10,11]. Increased awareness about the importance of bleedings in ACS patients has prompted the development of bleeding stratification risk scores to guide the implementation of preventive strategies [12]. In the recent years, various risk models have been developed and validated [13,14,15,16,17,18]. Although they showed overall a satisfactory performances regarding in-hospital and acute bleedings, the utility of these models to predict long-term bleeding is unclear [19]. The HAS-BLED score was initially developed to assess the bleeding risk in patients with AF receiving chronic anticoagulant therapy [20]. In these patients, it has also been shown to predict cardiovascular events and long-term outcome [21]. The observation by Pisters et al. [20] that HAS-BLED predictive efficacy was particularly high in patients receiving antiplatelet therapy led to its evaluation in predicting bleeding events and major acute cardiovascular events (MACE) in patients receiving dual antiplatelet therapy (DAPT) after percutaneous coronary interventions (PCI) and stenting with or without AF [22,23,24,25,26,27]. Moreover, the HAS-BLED score predictive performance was tested in patients with ACS receiving DAPT or triple antithrombotic therapy, showing moderate accuracy [28,29,30]. The principal objective of this study was to evaluate the predictive performance of the HAS-BLED risk score regarding in-hospital and long-term bleeding events and mortality in ACS patients without atrial fibrillation. In addition, we evaluated the effects of an alternative definition of renal dysfunction based on eGFR estimation on predictive accuracy of the risk model.
Materials and methods
Study population
Individuals admitted to the Coronary Care Unit (CCU) of San Paolo Hospital in Milan (Italy) between November 2012 and 2014 and between June 2016 and December 2017, with a final diagnosis of acute coronary syndrome (ACS), were considered for the study. The diagnosis of ACS was based on new onset symptoms consistent with cardiac ischemia plus at least one of the following objective criteria: electrocardiographic changes indicative of myocardial ischemia, troponin elevation above the 99th percentile threshold of a healthy reference population, with 10% coefficient of variability (Troponin I, Vitros ES assay, Ortho Clinical Diagnostics), previously documented coronary artery disease, defined as history of myocardial infarction, or previous angiographic demonstration of coronary stenosis ≥ 50%. Patients were classified as having ST-elevation myocardial infarction (STEMI) and non-ST-elevation ACS (NSTE-ACS) according to the standardized electrocardiographic criteria. The diagnosis of unstable angina required the absence of diagnostic elevation of troponin. All-comers design study was adopted with no restriction on age or on critically ill patient inclusion. The only exclusion criteria were a history of atrial fibrillation and the need of chronic anticoagulant therapy. A post-discharge 12-month follow-up was performed by phone call and/or ambulatory visits. This study was a retrospective analysis of prospectively collected data in a clinical registry database. The study complies with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of our Institution. All the patients signed a standard consent regarding sensitive personal data treatment.
Study design
Baseline clinical characteristics, medical history, biochemical and electrocardiographic findings, angiographic data, treatments administered during hospitalization, and incidence of in-hospital and out-of-hospital adverse events were collected on an electronic database (Microsoft Excel, Microsoft Office 2010 package) designed for ACS patients admitted to our CCU. In particular, in the database, all the elements pertinent to the definition of the HAS-BLED score were included (Table 1). Thus, for all the patients, the score was calculated based on admission clinical and laboratory data. Since patients with atrial fibrillation and/or the need of chronic anticoagulant therapy were excluded from the study, the item L, corresponding to the INR values trend, was set to 0 for all the patients. The score was calculated both using the renal dysfunction definition proposed in the original publication (that is, renal transplant/dialysis or serum creatinine ≥ 200 µmol/L or ≥ 2.26 mg/dL) [20], and by replacing it with an alternative renal dysfunction criteria based on eGFR calculation using the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) equation [31]. In particular, three alternative eGFR cut-off levels were analyzed: < 30, < 60, and ≤ 90 ml/min/1.73 mq, assigning one point for each alternative cut-off level. Moreover, for all the HAS-BLED scores obtained, patients were stratified into three bleeding risk categories (low risk: score = 0–1; moderate risk: score = 2; high risk: score ≥ 3). Finally, the analysis of the database allowed the determination of the incidence of in-hospital and post-discharge 12-month bleeding events and mortality. Bleedings were recorded in the database using the Bleeding Academic Research Consortium (BARC) standardized definition criteria [32].
Statistical analysis
Continuous variables are described as mean ± standard deviation (SD) or median and inter-quartile range (IQR), as appropriate. Categorical variables are described as absolute values and percentages. Comparisons between continuous variables were performed with Mann–Whitney test and ANOVA method as appropriate and associations between categorical variables were studied by the Chi-square test, the Fisher’s exact test, or the Cochran–Armitage Trend test, as appropriate. Risk models calibration was assessed by the Hosmer–Lemeshow test which determines how close is the correspondence between predicted and observed incidence of events [33]. In this test, a p < 0.05 indicates a lack of model adjustment. The discriminatory capacity of the risk models was assessed deriving their C-statistics, using receiving-operating characteristic (ROC) curves. The calibration and discrimination of the risk models were assessed with respect to in-hospital and post-discharge 12-month overall bleeding events (defined as BARC ≥ 2), in-hospital major bleeding events (defined as BARC 3 or 5; coronary artery bypass graft (CABG)-related bleedings were not considered in the study), and in-hospital and post-discharge mortality. The C-statistics of the risk models were compared using the DeLong’s test [34]. MedCalc Statistical Software version 16.2.0 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016) was used for all the statistical analysis. Statistical significance was defined as p < 0.05.
Results
Baseline characteristics
A total of 704 patients composed the final-study population. The demographic and clinical baseline characteristics of study patients and in-hospital treatments are shown in Table 2. Median age was 67 years (IQR 57–77) and 28% were females. In 48% of patients, the diagnosis was of NSTE-ACS and in 52% of STEMI. Regarding risk factors, 25% had a history of diabetes, 63% were affected by arterial hypertension, and 48% had dyslipidaemia. The GRACE score showed a median value of 131 (IQR 106–154). Most patients were treated with dual oral antiplatelet therapy, while only 27% received glycoprotein IIb/IIIa antagonists. In-hospital anticoagulant therapy was used in 90% of patients. Coronary angiography was performed in 97% of patients, using a vascular radial approach in 62% of cases, and a percutaneous coronary intervention (PCI) was performed in 77% of them. None of the patients included in the study was referred for urgent CABG. Finally, in 9 patients (1.3%), an intra-aortic balloon catheter was used. Of the 685 patients discharged from the hospital, 611 (89%) completed the post-discharge follow-up (mean value of 11.6 ± 0.8 months) and the characteristics of the follow-up population did not show statistically significant differences in comparison to the patients lost at follow-up, with the exception of an higher prevalence of arterial hypertension and dyslipidaemia (Table 3). In Table 4 are shown separately the mean values of HAS-BLED scores in the whole population and in the subgroup of patients that completed the follow-up period.
In-hospital and follow-up adverse events
As far as in-hospital period is concerned, 33 patients underwent a bleeding event, in 19 cases a BARC type 2 and in 14 cases a BARC type 3 bleeding. As a result, the incidence rate of overall bleedings was 4.7% and that of major bleedings was 2%. No fatal bleedings were observed. Considering overall bleedings, in 15% of cases, the event was related to the vascular access site, in 30% of cases to gastrointestinal bleeding, in 9% of cases to genitourinary bleeding, in 6% of cases to bronco-pulmonary bleeding, and in 40% of cases was related to multiple sites or undetected single site. Twenty-four patients (3.4%) underwent blood transfusions. In-hospital mortality was 2.7%. Regarding the follow-up period, the incidence rate of overall bleedings was 3.1%. In 52% of cases, the event was related to gastrointestinal bleeding, in 31% of cases to genitourinary bleeding, and in 17% of cases, it was not related to a single detectable site. In 12 patients, the bleeding event led to modification or discontinuation of dual antiplatelet therapy. No fatal bleedings were observed. Finally, out-of-hospital mortality was 4.4%. As shown in Table 5, the incidence of bleedings and of mortality rises across the risk categories for each of the HAS-BLED risk models considered, concerning both the in-hospital and follow-up periods, with significant results for the Cochran–Armitage test for trend, with the exception of in-hospital major bleedings and follow-up overall bleedings for HAS-BLED ≤ 90.
Risk score calibration and discrimination
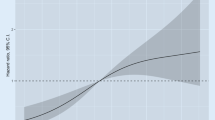
In Table 6 are summarized the calibrations and discriminations of each of the HAS-BLED risk models evaluated for in-hospital and follow-up overall bleedings, major bleedings, and mortality. As shown, the calibration of all the risk models was good, as demonstrated by the non-significant results of the Hosmer–Lemeshow test. The discriminatory capacity was moderate-to-good for all in-hospital and follow-up events considered (C-statistic from 0.65 to 0.68 for in-hospital overall bleedings, from 0.68 to 0.73 for in-hospital major bleedings, from 0.71 to 0.76 for in-hospital mortality (Fig. 1), from 0.65 to 0.66 for follow-up overall bleedings, and from 0.64 to 0.71 for follow-up mortality (Fig. 2)). No statistically significant differences were found between the discriminatory capacity of the risk models for all the events considered, with the exception of out-of-hospital mortality for which HAS-BLED model assuming an eGFR value < 60 ml/min/1.73 mq as criteria for renal dysfunction showed a discriminative performance significantly higher in comparison to the other risk models.
Receiver-operating characteristic curves of the HAS-BLED risk models for in-hospital bleeding events and mortality. a Overall bleedings, b major bleedings, and c mortality. C-statistic values are shown in Table 6
Receiver-operating characteristic curves of the HAS-BLED risk models for post-discharge bleeding events and mortality. a Overall bleedings; b mortality. C-statistic values are shown in Table 6
Discussion
The main results of the present study can be summarized as follows: (1) in our population, the HAS-BLED score demonstrated an overall moderate predictive accuracy for in-hospital and follow-up bleeding events, (2) the risk model also showed a fairly good predictive performance for in-hospital mortality, while the predictive accuracy was lower for post-discharge mortality, and (3) the utilization of eGFR-based alternative definitions of renal dysfunction resulted in a similar predictive accuracy for all the events considered, except when considering out-of-hospital mortality for which HAS-BLED with a < 60 ml/min/1.73 mq eGFR cut-off resulted in a significantly higher predictive performance.
Although the HAS-BLED score was established to predict bleeding and clinical outcomes in patients with AF receiving anticoagulant therapy, in the study of Pisters et al. [20], it showed a high predictive accuracy for bleeding events in the subgroup of patients on antiplatelet therapy. Based on this observation, the applicability of the HAS-BLED risk model has been investigated in patients receiving DAPT after PCI with or without AF. While two studies [22, 23] found that HAS-BLED score could not be used to assess the long-term risk of bleeding or MACE in AF patients undergoing PCI; in the PCI patients population without AF, the HAS-BLED score showed a fairly good predictive performance for the long-term occurrence of MACE and bleeding events [24,25,26,27]. A few studies were conducted in patients with ACS. Smith et al. [28] showed that HAS-BLED score significantly predicted the 1-year spontaneous bleeding events (C-statistic 0.67) in ACS patients receiving triple antithrombotic therapy and Hsieh et al. [29, 30] demonstrated that the HAS-BLED score show an high predictive accuracy for in-hospital major bleeding (C-statistic 0.80), no statistically different from that of CRUSADE and ACUITY-HORIZONS, and for 3-year survival (C-statistic 0.76), in ACS patients without AF.
Our study was conducted on a real-world population of ACS patients without AF. In our patients, the HAS-BLED score showed an adequate calibration and overall a moderate or fairly good predictive accuracy for in-hospital bleeding events (C-statistic of 0.68 for ≥ 2 BARC bleedings and of 0.71 for major bleedings) and mortality (C-statistic of 0.73), and for post-discharge 12-month bleeding events (C-statistic of 0.65 for ≥ 2 BARC bleedings), and mortality (C-statistic of 0.64). When compared with the studies by Hsieh et al. [29, 30], our results confirm the overall fairly good performance of HAS-BLED in predicting in-hospital bleeding events, although with lower C-statistic values, while a less satisfactory predictive accuracy was observed for long-term mortality. Our study differs in some aspects from those by Hsieh et al. Our study population was composed of non-selected patients with ACS, both of NSTE and STEMI types, while Hsieh studied only patients with non-ST-elevation myocardial infarction. Moreover, we evaluated in-hospital occurrence of both bleedings and mortality and post-discharge 1-year bleedings and mortality, while Hsieh considered only in-hospital major bleedings and 3-year mortality. Finally, in our population, almost 59% of patients received ticagrelor or prasugrel and 27% were treated with a glycoprotein IIb/IIIa antagonist, while all patients studied by Hsieh were on clopidogrel and none received a glycoprotein IIb/IIIa antagonist. Despite the wide utilization of more potent antiplatelet agents in our population, we observed a low 2% incidence of in-hospital major bleedings, with a 4.7% incidence of overall bleedings, figures largely lower than those observed by Hsieh et al. As pointed out in their study, the high incidence of in-hospital major bleeding (6.5%) which they observed might be explained, at least partially, by race-ethnic differences in responses to antiplatelet therapy observed in Asian patients [29, 35, 36]. Anyhow, the high incidence of in-hospital bleeding events can partially explain the higher predictive accuracy showed by HAS-BLED in their study. According to this, the high long-term mortality which they observed (15.4%) can partially explain the higher C-statistic that they obtained in comparison to our results regarding predictive accuracy of HAS-BLED for post-discharge mortality. As further aspects of differentiation, based on the observations pointing out a significant association also of minor grades of BARC bleedings with long-term mortality [10, 11, 37], we evaluated the predictivity of the HAS-BLED score not only for major bleedings but also for overall bleedings, that is ≥ 2 BARC grade bleedings. We observed a moderately good performance regarding in-hospital events and, more importantly, also for post-discharge bleedings (C-statistic of 0.65). We think that this result might be particularly useful in the clinical practice, since BARC type ≥ 2 bleedings were demonstrated to effectively predict interruption of long-term antiplatelet therapy [38] and risk scores specifically developed in ACS patients show overall only a modest predictive accuracy for post-discharge bleedings [19].
Renal insufficiency is associated with an increased risk of bleeding and it has been consistently found to be a powerful predictor of hemorrhagic complications in ACS patients, acting through a series of mechanisms of which platelet dysfunction, endothelial cells dysregulation, fibrinolytic system activation, and antithrombotic drugs overdosing/accumulation appear to be particularly relevant [12, 39]. Moreover, renal failure is associated with an increased risk of MACE and mortality in patients with ACS and in patients undergoing PCI [40, 41]. As a consequence of this, the majority of the risk models developed to predict bleeding complications and MACE includes a measure of renal function. The HAS-BLED risk model contains serum creatinine dosing to evaluate renal function and the presence of renal impairment is defined, besides renal transplant or dialysis, as serum creatinine ≥ 200 µmol/L (≥ 2.26 mg/dL) (Table 1) [20]. Although age is a component of the risk model, sex and race are not included, thus potentially producing inaccuracy in glomerular filtration rate estimation. Moreover, eGFR is widely accepted as a more accurate indicator of renal function. Based on these considerations, in our study, we evaluated if replacing the original creatinine-based renal impairment definition with an alternative renal dysfunction criteria based on eGFR calculation using the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) equation [31] might enhance the discriminative performance of HAS-BLED model. We tested separately three different eGFR cut-off criteria corresponding to mild, moderate, and severe definitions of chronic renal failure, that is, ≤ 90, < 60, and < 30 ml/min/1.73 mq. As shown in Table 4, no statistically significant differences were found between mean values of HAS-BLED scores obtained using the usual model and the < 30 eGFR model, both in the whole population and in the post-discharge population, suggesting that, in our population, the creatinine cut-off used in the original HAS-BLED model corresponds roughly to a condition of severe renal failure as obtained using the eGFR-based measurement. The utilization of less restrictive criteria for renal impairment, that is ≤ 90 and < 60 ml/min/1.73 mq eGFR, resulted in significantly higher HAS-BLED mean values, due to the allocation of more patients in the higher bleeding risk categories, as shown in Table 5. When compared to the original HAS-BLED, all the alternative eGFR-based models showed a good calibration with a similar predictive accuracy for bleeding events, both in-hospital and post-discharge. As shown in Table 6, no statistically significant differences were found between the C-statistic of the various risk models for all the bleeding events considered. On the other hand, the risk model incorporating the < 60 ml/min/1.73 mq eGFR definition of renal impairment showed, in comparison to the original HAS-BLED, a significantly higher predictive accuracy for post-discharge mortality (C-statistic 0.71 vs 0.64) and a not significant trend for in-hospital mortality (C-statistic 0.76 vs 0.73), suggesting that this threshold for renal impairment ensures a more favorable accuracy profile, combining a fairly good accuracy for bleeding events with the best one for mortality. Until now, we know no other studies in literature on this topic. However, the results which we obtained are in agreement with observations, suggesting that, in patients undergoing surgery, postoperative mortality rises more steeply once eGFR falls below 60 ml/min/1.73 mq [42].
Limitations of the study
The present study presents some limitations. The first one is the relatively limited sample size and the consequent small number of events. This did not allow the evaluation of the risk model performance in patients’ subgroups, such as STEMI and NSTE-ACS patients as an example. Other limitations are that it was designed as a retrospective analysis of prospectively collected data from a clinical registry, including limitations inherent to such study designs, and that it is a single-institution experience, limiting the generalizability of our findings to other populations. However, we think that our population, although relatively small, is well balanced, representing a contemporary ACS population, almost equally subdivided between STEMI and NSTE-ACS cases and managed according to current clinical practice, with the great majority of patients referred to coronary angiography, 77% to percutaneous coronary interventions, and the majority being treated with new P2Y12 agents.
Conclusions
The conclusions that we can draw from our study can be summarized in two main points. First, the HAS-BLED score seems to perform reasonably well in ACS patients without AF to predict in-hospital and post-discharge bleeding events and in-hospital mortality. This might be particularly useful, because, using a single simple score, easier to calculate than other bleeding and MACE oriented scores, we can predict with moderate-to-good accuracy both overall in-hospital main adverse events and post-discharge hemorrhagic complications. Second, the utilization of eGFR with a cut-off value of < 60 ml/min/1.73 mq as the threshold for renal impairment definition seems to enhance the predictive performance of the risk model, adding a better discriminative capacity for post-discharge mortality.
References
Eikelboom JW, Metha SR, Anand SS, Xie C, Fox KA, Yusuf S (2006) Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 114:774–782
Kadakia MB, Desai NR, Alexander KP, Chen AY, Foody JM, Cannon CP, Wiviott SD, Scirica BM (2010) Use of anticoagulant agents and risk of bleeding among patients admitted with myocardial infarction: a report from the NCDR ACTION Registry_GWTG. JACC Cardiovasc Interv 3:1166–1177
Fox KA, Carruthers K, Steg PG, Avezum A, Granger CB, Montalescot G, Goodman SG, Gore JM, Quill AL, Eagle KA (2010) Has the frequency of bleeding changed over time for patients presenting with an acute coronary syndrome? The global registry of acute coronary events. Eur Heart J 31:667–675
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS (2015) Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 372:1791–1800
Valgimigli M, Costa F, Byrne R, Haude M, Baumbach A, Windecker S (2015) Dual antiplatelet therapy duration after coronary stenting in clinical practice: results of an EAPCI survey. EuroIntervention 11:68–74
Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA (2005) Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol 96:1200–1206
Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB 3rd, Ohman EM, Stone GW (2007) Impact of major bleeding on 30-days mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY trial. J Am Coll Cardiol 49:1362–1368
Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schömig A, Kastrati A (2008) Periprocedural bleeding and 1-year outcome after percutaneous interventions: appropriateness of including bleeding as a component of a quadruple end-point. J Am Coll Cardiol 51:690–697
Suh JW, Mehran R, Claessen BE, Xu K, Baber U, Dangas G, Parise H, Lansky AJ, Witzenbichler B, Grines CL, Guagliumi G, Kornowski R, Wöhrle J, Dudek D, Weisz G, Stone GW (2011) Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous intervention in acute myocardial infarction. The HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) Trial. J Am Coll Cardiol 58:1750–1756
Vranckx P, White HD, Huang Z, Mahaffey KW, Armstrong PW, Van de Werf F, Moliterno DJ, Wallentin L, Held C, Aylward PE, Cornel JH, Bode C, Huber K, Nicolau JC, Ruzyllo W, Harrington RA, Tricoci P (2016) Validation of BARC bleeding criteria in patients with acute coronary syndromes. The TRACER Trial. J Am Coll Cardiol 67:2135–2144
Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, Van de Werf F, Harrington RA, Mahaffey KW, Tricoci P (2017) Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J 38:804–810
Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D, Hamon M, Helft G, Fox KAA, Kristensen SD, Rao SV, Verheugt FWA, Widimsky P, Zeymer U, Collet JP (2011) Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 32:1854–1864
Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP (2009) Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcome with Early implementation of the ACC/AHA Guidelines) bleeding score. Circulation 119:1873–1882
Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW (2010) A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 55:2556–2566
Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, Della Riva D, Fahy M, Xu K, Stone GW (2012) A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: the ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage strategY-Percutaneous Coronary Intervention) risk score. JACC Cardiovasc Interv 5:1108–1116
Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP (2011) In-hospital major bleeding during ST-elevation and non-ST-elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry(R)-GWTG. Am J Cardiol 107:1136–1143
Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzembichler B, Weisz G, Steg PG, Pocock S (2016) Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk score from PARIS. J Am Coll Cardiol 67:2224–2234
Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M (2017) Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 389:1025–1034
Ko SQ, Valsdottir LR, Strom JB, Cheng YC, Hirayama A, Liu PH, Yanagisawa N, Yen H, Shen C, Yeh RW (2018) Meta-analysis of bleeding risk prediction scores in patients after percutaneous coronary intervention on dual platelet therapy. Am J Cardiol 122:1843–1852
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH (2010) A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 138:1093–1100
Gallego P, Roldan V, Torregrossa JM, Galvez J, Valdes M, Vicente V, Marin F, Lip GY (2012) Relation of the HAS-BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol 5:312–318
Kiviniemi T, Puurunen M, Schlitt A, Rubboli A, Karjalainen P, Vikman S, Niemela M, Lathela H, Lip GY, Airaksinen KE (2014) Performance of bleeding risk-prediction scores in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol 113:1995–2001
Puurunen MK, Kiviniemi T, Schlitt A, Rubboli A, Dietrich B, Karjalainen P, Nyman K, Niemela M, Lip GY, Airaksinen KE (2014) CHADS2, CHA2DS2-Vasc and HAS-BLED as predictors of outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention. Thromb Res 133:560–566
Konishi H, Miyauchi K, Tsuboi S, Ogita M, Naito R, Dohi T, Kasai T, Tamura H, Okazaki S, Isoda K, Daida H (2015) Impact of the HAS-BLED score on long-term outcomes after percutaneous coronary intervention. Am J Cardiol 116:527–531
Costa F, Tijssen JG, Ariotti S, Giatti S, Moscarella E, Guastaroba P, De Palma R, Andò G, Oreto G, Zijlstra F, Valgimigli M (2015) Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc 4:e002524
Capodanno D, Rossini R, Musumeci G, Lettieri C, Senni M, Valsecchi O, Angiolillo DJ, Lip GYH (2015) Predictive accuracy of CHA2DS2-VASc and HAS-BLED scores in patients without atrial fibrillation undergoing percutaneous coronary intervention and discharged on dual antiplatelet therapy. Int J Cardiol 199:319–325
Shah RR, Pillai A, Omar A, Zhao J, Arora V, Kapoor D, Poommipanit P (2017) Utility of HAS-BLED score in risk stratifying patients on dual antiplatelet therapy post 12 months after drug-eluting stent placement. Cath Cardiovasc Interv 89:E99–E103
Smith JG, Wieloch M, Koul S, Braun OO, Lumsden J, Rydell E, Ohman J, Schersten F, Svensson PJ, van der Pals J (2012) Triple antithrombotic therapy following an acute coronary syndrome: prevalence, outcomes and prognostic utility of the HAS-BLED score. EuroIntervention 8:672–678
Hsieh MJ, Wang CC, Chen CC, Wang CL, Wu LS, Hsieh IC (2015) HAS-BLED score predicts risk of in-hospital major bleeding in patients with acute non-ST segment elevation myocardial infarction. Thromb Res 136:775–780
Hsieh MJ, Lee CH, Chen CC, Chang SH, Wang CY, Hsieh IC (2017) Predictive performance of HAS-BLED risk score for long-term survival in patients with non-ST elevated myocardial infarction without atrial fibrillation. J Cardiol 69:136–143
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus from the Bleeding Academic Research Consortium. Circulation 123:2736–2747
Lemeshow S, Hosmer D (1982) A review of goodness of fit statistic for use in the development of logistic regression models. Am J Epidemiol 115:92–106
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Mak KH, Bhatt DL, Shao M, Hankey GJ, Easton JD, Fox KA, Topol EJ (2009) Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J 157:658–665
Saunders E, Ofili E (2008) Epidemiology of atherothrombotic disease and the effectiveness and riskd of antiplatelet therapy: race and ethnic considerations. Cardiol Rev 16:82–88
Ndrepepa G, Schuster T, Hadamitzky M, Byrne RA, Mehilli J, Neumann FJ, Richardt G, Schulz S, Laugwitz KL, Massberg S, Schomig A, Kastrati A (2012) Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation 125:1424–1431
Choi JH, Seo JM, Lee DH, Park K, Kim YD (2015) Clinical utility of new bleeding criteria: a prospective study of evaluation for the Bleeding Academic Research Consortium definition of bleeding in patients undergoing percutaneous coronary intervention. J Cardiol 65:324–329
Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K (2014) Haemostasis in chronic kidney disease. Nephrol Dial Transpl 29:29–40
Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Piñero G, Avezum A, Gulba D, Esteban J, Gore JM, Johnson J, Gurfinkel EP, Investigators GRACE (2003) Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the Global Registry of Acute Coronary Events (GRACE). Heart 89:1003–1008
Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB (2002) The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol 39:1113–1119
Mooney JF, Ranasinghe I, Chow CK, Perkovic V, Barzi F, Zoungas S, Holzmann MJ, Welten GM, Biancari F, Wu VC, Tan TC, Cass A, Hillis GS (2013) Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta-analysis. Anesthesiology 118:809–824
Acknowledgements
The authors acknowledge the “Centro di Ricerca Aldo Ravelli” for supporting the study. We specify that they did not have any role in the design or conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation of the manuscript, or the decision to publish.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors state that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castini, D., Persampieri, S., Sabatelli, L. et al. Utility of the HAS-BLED score for risk stratification of patients with acute coronary syndrome. Heart Vessels 34, 1621–1630 (2019). https://doi.org/10.1007/s00380-019-01405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01405-1