Abstract
This study investigated the safety and efficacy of a sustained release of basic fibroblast growth factor (bFGF) with biodegradable gelatin hydrogel sheets as therapeutic angiogenesis in canine chronic myocardial infarction (MI) models. Canine chronic MI model was induced by ligating the left anterior descending coronary artery and its diagonal branches. At 4 week post-induction, we applied either saline (Control group, n = 5) or 200 μg of bFGF (Treatment group, n = 6) soaked gelatin hydrogel sheets on the ischemic area of the left ventricular (LV) wall. At 6 weeks after the procedure, we evaluated the efficacy by echocardiography and immunohistochemical study. There were no procedure-related adverse events or deaths. The serum bFGF level was under detectable levels in all animals at any sampling points. In terms of efficacy, echocardiographic evaluation demonstrated that fractional shortening was significantly improved in the treatment group. In addition, immunohistochemical study showed that the capillary density in the border zone of the MI area, as well as the MI area, significantly increased in the treatment group. Therapeutic angiogenesis by bFGF using biodegradable gelatin hydrogel sheets was safe, increased the capillary density, and improved LV function in canine chronic MI models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease remains one of the major causes of death worldwide [1, 2]. The concept of “therapeutic angiogenesis” (i.e., the administration of growth factors, gene therapy, or cell transplantation) has emerged as a new therapeutic option for severe ischemic conditions resistant to conventional surgical and/or catheter-based revascularization, and proof-of-concept pre-clinical studies using experimental animal models and clinical trials have been conducted with favorable results [3, 4]. The purpose of therapeutic angiogenesis is to increase the blood perfusion of the microenvironment which may mitigate the ischemic condition, reduce the tissue damage, and ameliorate the ischemia-related dysfunction.
Basic fibroblast growth factor (bFGF) [5] is known to promote proliferation of mesenchymal cells and potently induce neovascularization which holds potential for the therapeutic angiogenesis. Recombinant human bFGF has been approved for clinical use and widely adopted for the treatment of skin ulcers in Japan [6]. However, the short biological half-life of bFGF in the body hampers its clinical application. To overcome the problem, we have developed a novel drug delivery system (DDS) using a biodegradable acidic gelatin hydrogel [7]. Gelatin is a water-soluble biopolymer originating from collagen and has been clinically used in various applications because of its non-toxic nature. Gelatin possesses a biodegradable feature and is gradually absorbed in the body by releasing binding substrates, allowing sustained release of the substrate [8, 9]. bFGF-incorporated gelatin hydrogel gradually releases bFGF in the body after administration which serves as a novel DDS to allow the long-lasting local release of bFGF at the applied region. We have previously demonstrated the effectiveness of bFGF-incorporated gelatin hydrogel in various animal models [10] and clinical studies [11, 12] for peripheral arterial disease (PAD) patients, such as atherosclerosis obliterans (ASO) or thromboangiitis obliterans (TAO) (Buerger’s disease) suffering from critical limb ischemia (CLI). Expanding the therapeutic angiogenesis to ischemic heart diseases would further mitigate the healthcare issues related to atherosclerosis.
The aim of this study was to investigate the safety and efficacy of the bFGF-incorporated gelatin hydrogel sheets transplantation on the ischemic myocardium in chronic canine myocardial infarction (MI) model.
Materials and methods
Animal preparation
We used male canines in this study (beagles, 14–16 months old, weighing 10–14 kg; Kitayama labs, Co., Ltd., Iwakuni, Japan). The animal experiments were performed at Shin Nippon Biomedical Laboratories (SNBL) (Kagoshima, Japan). The procedure protocol for animals was approved by the Institutional Animal Care and Use Committee of SNBL. All animals received humane care in compliance with standards published by the National Research Council (Guide for the Care and Use of Laboratory Animals, NIH OACU) of the National Institutes of Health Policy on Human Care and Use of Laboratory Animals. In compliance with these standards, every effort was made to minimize the pain and discomfort of experimental animals.
Preparation of bFGF-incorporated gelatin hydrogel sheets
Human recombinant bFGF with an isoelectric point of 9.6 was commercially available (Kaken Pharmaceutical Co, Tokyo, Japan). A gelatin sample with an isoelectric point of 5.0 was isolated from the porcine skin tissue through the alkaline process (Nitta Gelatin Co, Osaka, Japan). Gelatin hydrogel sheets were prepared in an aseptic room as previously described [13]. Briefly, gelatin hydrogel was prepared through the glutaraldehyde cross-linking of gelatin in an aqueous solution. The resulting hydrogel was soaked in an aqueous solution of glycine for 3 h to block free aldehyde groups in the hydrogel. The concentrations of gelatin hydrogel, glutaraldehyde, and glycine were 4.5%, 0.083% and 100 mM, respectively. The temperature for soaking in glycine was 37 °C on a shaker. We made the gelatin hydrogel sheets with a mold. The thickness of the gelatin sheet was around 2 mm. These gelatin hydrogel sheets are formed in the approximately same thickness. The temperature of crosslink the gelatine hydrogel was 4 °C. They were washed with double distilled water and cut in 2 × 4 cm. After they were freeze-dried, gelatin hydrogel sheets were packed in sterile bags. To incorporate bFGF into the gelatin hydrogel sheets, an aqueous solution of bFGF (100 µg/ml) was carefully dropped onto freeze-dried sheets, and they were then stored at ambient temperature for 1 h as previously reported [14]. The gelatin hydrogel sheets were designed to slowly release bFGF in animal bodies for approximately 3–4 weeks after treatment which is certified by previous in vitro and in vivo studies [7, 14,15,16]. A positively charged protein drug (bFGF this time) is electrically complexed with negatively charged polymer chains, consisting a carrier matrix. The degradation of the polymer carrier itself will lead to drug release. We can control the speed of the degradation as long as 4 weeks which may contribute to the prolonged release of bFGF [8]. Production of the gelatin hydrogel sheets was performed in accordance with Good Manufacturing Practice (GMP) standards.
MI induction and implantation of bFGF-incorporated gelatin hydrogel sheets
We evaluated the efficacy of the therapy in canine with chronic myocardial infarction (MI) model (Fig. 1). After sedation with intramuscular administration of dexmedetomidine (0.08 mg/kg), midazolam (0.4 mg/kg), and atropine (0.01 mg/kg), dogs were intubated. General anesthesia was maintained with 0.5–5% isoflurane in oxygen. End-tidal PaCO2, heart rate, electrocardiogram, and blood pressure were continuously monitored. Respiratory rates and concentration of isoflurane were controlled by a mechanical ventilator. Dogs were positioned in right lateral recumbency. After induction of anesthesia, a left thoracotomy was performed through the fourth intercostal space and pericardiotomy was made. MI was induced by ligating the proximal left anterior descending coronary artery just distal to the first diagonal branch and the first to the third diagonal branches. Then, the pericardium was approximated and chest was closed in layers. Induction of MI in canines yielded approximately 20% mortality rate within 24 h of the induction.
Experimental protocol. Induction of MI, ligation of the proximal left anterior descending coronary artery just distal to the first diagonal branch, and the first to third diagonal branches. Treatment, gelatin hydrogel sheets with saline (n = 5) or bFGF (200 μg, n = 6) were applied to the infarction area of the left ventricular wall
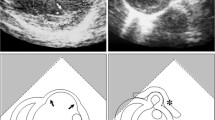
Four weeks after MI induction, 11 survived canines with transmural MI (evaluated by echocardiography) were randomly divided into two groups: the control group (gelatin hydrogel sheets with saline, n = 5) and the bFGF group (gelatin hydrogel sheets with 200 μg of bFGF, n = 6). Re-do left thoracotomy was performed through the sixth or seventh intercostal space under aforementioned general anesthesia, and pericardiotomy was made. In each group, gelatin hydrogel sheets with either saline or bFGF were applied to the infarction area of the left ventricular (LV) wall (Fig. 2). To prevent displacement of the sheets, these gelatin hydrogel sheets were covered with 0.1 mm polytetrafluoroethylene sheet (Gore-Tex Surgical Membrane, W.L. Gore and Assoc, Inc. Flagstaff, AZ, USA) and secured with sutures, then, the pericardium was loosely approximated and the thoracotomy was closed in layers.
Measurement of serum bFGF levels
In all canines, 2 ml of blood was sampled at 3 and 6 weeks after the procedure to measure the serum bFGF level with enzyme-linked immunosorbent assay (ELISA) using a high-sensitivity kit (Human FGF basic Immunoassay, R&D Systems, Inc. Minneapolis, MN, USA) (Fig. 1) according to the manufacturer’s instruction.
Echocardiographic evaluation
Echocardiographic measurements were performed with a 12-MHz ultrasound transducer (connected with SONOS 7500 Ultrasound System, Philips Healthcare, Bothell, WA, USA) before and 4 weeks after the MI induction, and 3 and 6 weeks after the treatment (Fig. 1). All echocardiographic parameters were measured by at least three consecutive cardiac cycles. LV end-diastolic dimension (LVEDd) and area (LVEDA), and end-systolic dimension (LVESd) and area (LVESA) were measured in the two-dimensional mode in the short-axis views at the level of the largest ventricular diameter. LVEDd and LVEDs are used to calculate LV fractional shortening (LVFS) by the following formula; LVFS (%) = (LVEDd − LVESd)/LVEDd × 100. LVEDA and LVESA were measured to calculate the percentage of LV fractional area change (LVFAC) using the following formula: LVFAC (%) = (LVEDA − LVESA)/LVEDA × 100.
Histological study
All canines were euthanized at 6 weeks after the treatment (Fig. 1). Subsequently, the heart and other organs were soaked and preserved in 10% buffered formalin solution. The transmural sections were stained with Azan staining and anti-factor VIII antibody (Agilent Technologies, Inc. Santa Clara, CA, USA). The fibrotic area was visualized by an all-in-one microscope (BZ-9000, Keyence, Osaka, Japan), then measured and calculated by BZ-II analysis application (Keyence). To evaluate the density of capillaries, the number was counted in random ten fields per slide (100× magnification) in the peri-infarct, infarct, and LV surface region of each section. The density of capillaries was defined as the number of vessels per mm2 containing smooth muscle cells detected by immunohistochemical staining (capillary size was < 25 μm in diameter) [17].
Data analysis
All values were shown as mean ± SD. The groups were compared by unpaired Student’s t test or analysis of variance (ANOVA) with Tukey’s test as post hoc. Statistical analyses were performed with JMP® 9 (SAS Institute Inc., Cary, NC, USA). Values of p < 0.05 were considered statistically significant.
Results
Safety of bFGF-incorporated gelatin hydrogel sheet implantation
There were no treatment-related adverse events or deaths. Procedure-related significant changes of clinical signs, body weight, and food consumption were not identified. The serum bFGF levels were under detectable in all animals at any sampling points.
Therapeutic effects of bFGF-incorporated gelatin hydrogel sheet implantation
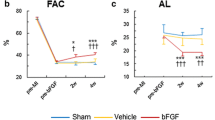
The results of echocardiographic evaluation are shown in Table 1. LVFS and LVFAC in control group did not significantly change after treatment compared to that in pretreatment (Pretreatment vs 3 vs 6 weeks; LVFS: 13.9 ± 0.8 vs 13.9 ± 1.3 vs 11.9 ± 0.9%; p = 1.0000 vs 3 weeks, 0.3068 vs 6 weeks/LVFAC: 24.0 ± 2.4 vs 24.8 ± 2.0 vs 22.7 ± 2.3%; p = 0.9994 vs 3 weeks, 0.9933 vs 6 weeks). However, LVFS and LVFAC in bFGF group after treatment were significantly higher at both 3 and 6 weeks compared to the values of pretreatment (LVFS: 13.6 ± 0.7 vs 18.3 ± 1.2 vs 18.6 ± 0.8%; p = 0.0004 vs 3 weeks, p = 0.0002 vs 6 weeks/LVFAC: 25.1 ± 2.2 vs 33.2 ± 1.8 vs 34.0 ± 2.1%; p = 0.0136 vs 3 weeks, p = 0.0061 vs 6 weeks). The value of LVFS and LVFAC was significantly higher in treatment group 6 weeks after surgery compared to those in control group (LVFS: p = 0.0019; LVFAC: p = 0.0158).
bFGF-incorporated gelatin hydrogel sheet increased vascular density
The immunohistochemical study demonstrated that vascular formation in the bFGF group at 6 weeks after treatment was more prominent compared to that observed in the control group (Fig. 3). We quantified the vascular density at peri-infarct and infarct zone, respectively. At the peri-infarct zone, the number of capillaries (196.8 ± 38.3 vessels/mm2 in the bFGF group, vs 110.6 ± 20.0 vessels/mm2 in the control group, p < 0.01) was significantly higher in the bFGF group than those in the control group (Fig. 4a). At the infarct zone, the number of capillaries was significantly higher in the bFGF group than that in the control group (155.5 ± 26.5 vs 96.2 ± 22.6 vessels/mm2, p < 0.05) (Fig. 4b). At the LV surface area just underneath, where the gelatin hydrogel sheets were applied, the number of capillaries was significantly higher in the bFGF group compared to that in the control group (136.6 ± 27.9 vs 72.9 ± 17.1 vessels/mm2, p < 0.05). We further evaluated fibrotic area by Azan staining. There was no significant difference in terms of the fibrotic area in both groups (12.1 ± 2.3 vs 13.3 ± 2.1%, p = 0.70).
Discussion
In the current study, we investigated the efficacy of bFGF with biodegradable gelatin hydrogel sheets as therapeutic angiogenesis in a canine chronic MI model. We found that bFGF incorporated into biodegradable gelatin hydrogel sheets significantly increased the capillary density in the ischemic heart and improved the LV systolic function after the procedure.
A large number of patients with coronary artery disease (CAD) suffer from heart failure, and LV function is a robust prognostic predictor in patients with CAD [18]. Although revascularization in such patients with LV dysfunction may relieve the symptoms and improve the survival rate [19], patients with extremely advanced pathology would not be able to benefit from the revascularization therapy due to typically diffused and severe atherosclerotic lesion of coronary arteries. Our method in the present study simply requires direct application of the bFGF-incorporated gelatin hydrogel sheets on the target area which can be applied for patient resistant to conventional revascularization.
We have previously conducted clinical trials of sustained release of bFGF with biodegradable gelatin hydrogel as therapeutic angiogenesis for patients with critical limb ischemia [11, 12]. The extension of this technology toward CAD would be beneficial for the healthcare of cardiovascular diseases, and the present proof-of-concept study would serve as a technological basis of therapeutic angiogenesis for CAD.
There are several reported approaches for therapeutic angiogenesis on animal ischemic heart models using viral vectors expressing vascular endothelial cell growth factor [20], fibrin glue incorporated with bFGF [21], or bFGF with polyvinyl alcohol–dextran blend hydrogel [22]. The main advantage of gelatin hydrogel as a DDS would be the safety of the treatment. The topical application of the hydrogel mediating a sustained release of cytokines would hamper the toxic side effects caused by the contamination of the cytokines within the systemic blood flow. In the present study, we did not observe toxic concentration of serum bFGF. On the other hand, the biological adverse reactions toward the gelatin hydrogel should be carefully assessed because of prolonged existence of the substrate in vivo.
In terms of the mechanisms of the myocardial recovery after therapeutic angiogenesis, there have been several investigational studies to date. Unger et al. reported that angiogenesis provides modest nutritive blood flow to a collateral-dependent region [23]. bFGF with gelatin hydrogel sheets may induce collateral circulation surrounding the MI area by angiogenesis, which is expected to improve the overall myocardial function. On the other hand, we could not observe the attenuation of fibrosis mediated by the bFGF treatment in the present study. Shudo et al. reported that one possible mechanism of the effect of skeletal myoblast (SMB) sheets is that the SMB sheet induced the development of the microvasculature, which resumed the hibernating myocardium [24, 25], thereby enhanced the recovery of myocardial performance [26]. Chronic hibernation develops in response to the episodes of myocardial ischemia–reperfusion or reduced blood flow [25]. It is reported that the functional recovery after revascularization is limited when the myocardial structure is severely damaged [18, 27]. When myocardial hibernation was formed through chronically reduced blood flow, increased blood flow could improve LV function. Although fibrotic area of myocardium caused by MI was not affected by bFGF treatment in the present study, bFGF with gelatin hydrogel sheets induced collateral development as angiogenesis which may account for the improvement of LV function through the recovery from myocardial hibernation accompanied by increased neovascularization.
Our study holds several limitations. First, our MI model was induced by experimental coronary artery ligations which did not fully recapitulate bona fide etiology of the progression of chronic ischemia of human heart which is highly related to atherosclerosis. Second, the follow-up period in the present study was 6 weeks after treatment. A longer observational period would be preferable to evaluate long-term effect and safety of this method. Third, we did not test multiple dose regimen of bFGF in the present study. We aim to evaluate the safety and effectiveness of multiple doses of bFGF in pre-clinical studies including no-treatment group in our future work. Fourth, we did not evaluate the invasion of macrophages and other inflammatory cells at the implanted site which should also be evaluated in our next work.
In conclusion, therapeutic angiogenesis by bFGF using biodegradable gelatin hydrogel sheets was safe and therapeutically effective for the improvement of LV function in canine chronic MI models. Further clinical studies are warranted for broad application of this technology.
References
World Health Organization (WHO) Global Health Estimates (GHE): cause of death, 2000–2012. Secondary World Health Organization (WHO) Global Health Estimates (GHE): cause of death, 2000–2012. http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. Accessed 3 Apr 2018
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S (2017) Heart disease and stroke statistics-2017 update: A Report From the American Heart Association. Circulation 135:e146–e603
Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K, Landmesser U, Mummery CL, Janssens S, Willerson J, Eschenhagen T, Ferdinandy P, Sluijter JP (2016) Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 37:1789–1798
Masumoto H, Sakata R (2012) Cardiovascular surgery for realization of regenerative medicine. Gen Thorac Cardiovasc Surg 60:744–755
Gospodarowicz D (1974) Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 249:123–127
Akita S, Akino K, Imaizumi T, Hirano A (2005) A basic fibroblast growth factor improved the quality of skin grafting in burn patients. Burns 31:855–858
Tabata Y, Nagano A, Ikada Y (1999) Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 5:127–138
Ikada Y, Tabata Y (1998) Protein release from gelatin matrices. Adv Drug Deliv Rev 31:287–301
Kushibiki T, Tomoshige R, Fukunaka Y, Kakemi M, Tabata Y (2003) In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J Control Release 90:207–216
Doi K, Ikeda T, Marui A, Kushibiki T, Arai Y, Hirose K, Soga Y, Iwakura A, Ueyama K, Yamahara K, Itoh H, Nishimura K, Tabata Y, Komeda M (2007) Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessels 22:104–108
Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, Saji Y, Inui K, Hashida T, Yokoyama S, Onodera R, Ikeda T, Fukushima M, Komeda M (2007) A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I–IIa study. Circ J 71:1181–1186
Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, Tanaka S, Yanagi S, Ito-Ihara T, Ikeda T, Murayama T, Teramukai S, Katsura T, Matsubara K, Kawakami K, Yokode M, Shimizu A, Sakata R (2016) Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels 31:713–721
Iwakura A, Tabata Y, Koyama T, Doi K, Nishimura K, Kataoka K, Fujita M, Komeda M (2003) Gelatin sheet incorporating basic fibroblast growth factor enhances sternal healing after harvesting bilateral internal thoracic arteries. J Thorac Cardiovasc Surg 126:1113–1120
Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y (1999) Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed 10:79–94
Tabata Y, Nagano A, Muniruzzaman M, Ikada Y (1998) In vitro sorption and desorption of basic fibroblast growth factor from biodegradable hydrogels. Biomaterials 19:1781–1789
Tabata Y, Ikada Y (1999) Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials 20:2169–2175
Uchida Y, Yanagisawa-Miwa A, Nakamura F, Yamada K, Tomaru T, Kimura K, Morita T (1995) Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J 130:1182–1188
Cornel JH, Bax JJ, Elhendy A, Maat AP, Kimman GJ, Geleijnse ML, Rambaldi R, Boersma E, Fioretti PM (1998) Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol 31:1002–1010
Trachiotis GD, Weintraub WS, Johnston TS, Jones EL, Guyton RA, Craver JM (1998) Coronary artery bypass grafting in patients with advanced left ventricular dysfunction. Ann Thorac Surg 66:1632–1639
Lazarous DF, Shou M, Stiber JA, Hodge E, Thirumurti V, Goncalves L, Unger EF (1999) Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res 44:294–302
Nie SP, Wang X, Qiao SB, Zeng QT, Jiang JQ, Liu XQ, Zhu XM, Cao GX, Ma CS (2010) Improved myocardial perfusion and cardiac function by controlled-release basic fibroblast growth factor using fibrin glue in a canine infarct model. J Zhejiang Univ Sci B 11:895–904
Fathi E, Nassiri SM, Atyabi N, Ahmadi SH, Imani M, Farahzadi R, Rabbani S, Akhlaghpour S, Sahebjam M, Taheri M (2013) Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med 7:697–707
Unger EF, Sheffield CD, Epstein SE (1990) Creation of Anastomoses between an extracardiac artery and the coronary circulation—proof that myocardial angiogenesis occurs and can provide nutritional blood-flow to the myocardium. Circulation 82:1449–1466
Braunwald E, Rutherford JD (1986) Reversible ischemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol 8:1467–1470
Heusch G, Schulz R, Rahimtoola SH (2005) Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol 288:H984–H999
Shudo Y, Miyagawa S, Fukushima S, Saito A, Shimizu T, Okano T, Sawa Y (2011) Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg 142:1188–1196
Maes A, Flameng W, Nuyts J, Borgers M, Shivalkar B, Ausma J, Bormans G, Schiepers C, De Roo M, Mortelmans L (1994) Histological alterations in chronically hypoperfused myocardium. Correlation with PET findings. Circulation 90:735–745
Acknowledgements
This work was supported by research grants from the Ministry of Health, Labor and Welfare, Japan [Grant no. 201409024A] (to R.S.) and Invited Research Project of Translational Research Center, Kyoto University Hospital (to R.S.). We thank Dr. Hemant Poudyal (Kyoto University) for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Kumagai, M., Minakata, K., Masumoto, H. et al. A therapeutic angiogenesis of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel sheets in a canine chronic myocardial infarction model. Heart Vessels 33, 1251–1257 (2018). https://doi.org/10.1007/s00380-018-1185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1185-6