Abstract
The aim of the study was to evaluate systemic right ventricular (RV) dyssynchrony in patients with congenitally corrected transposition of the great arteries (CCTGA) and transposition of the great arteries (TGA) with New York Heart Association functional class (NYHA FC) < III. We used cardiac magnetic resonance (CMR) to evaluate the dyssynchrony and assessed whether RV dyssynchrony can be predictive of major cardiac events in their early stages in these patients. We enrolled 71 consecutive, NYHA FC < III patients with systemic RV who underwent CMR between April 1995 and December 2016. We measured intra- and inter-ventricular dyssynchrony using a feature-tracking method of cine magnetic resonance imaging. The predictors of major cardiac events were analyzed using the Cox hazard analysis. The data from 36 patients with CCTGA and 35 patients with TGA after an atrial switch were analyzed. Seven (19.4%) patients with CCTGA and 6 (17.1%) patients with TGA showed a QRS duration of ≥ 130 ms. There were significant intra- and inter-dyssynchrony in the systemic RV groups, compared to healthy controls. The average follow-up period was 5.1 ± 3.9 years. From among patients with CCTGA, 9 (25.0%) had major cardiac events. The parameters including NYHA FC, indexed RV volume, longitudinal early diastolic strain rate, and intra- and inter-ventricular dyssynchrony were predictive of major cardiac events. From among patients with TGA, 12 (34.3%) had major cardiac events. Age, NYHA FC, QRS duration, RV volume, RV mass index, LV volume, global longitudinal/circumferential strain and intraventricular dyssynchrony, were all predictive of major cardiac events. Systemic RV in NYHA FC < III patients with CCTGA and TGA, have obvious intra- and inter-dyssynchrony, suggesting ineffective wall motion and potential RV dysfunction. Intraventricular dyssynchrony can be an adjunct predictor of major cardiac events in mildly symptomatic patients with both CCTGA and TGA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic right ventricular (RV) dyssynchrony is one of the prognostic factors in congenitally corrected transposition of the great arteries (CCTGA) and transposition of the great arteries (TGA) after an atrial switch [1]. The contraction of the systemic RV is easily reduced by various factors because the RV has the potential for ischemia and lacks effective torsion [2,3,4]. Therefore, synchronous RV wall motion is essential to compensate for these weaknesses and to maintain an effective cardiac output. Because of sinus nodal and/or conduction issues, these patients often need pacemaker implantation. However, RV function often deteriorates rapidly after pacemaker implantation [5, 6] mainly because of pacemaker-induced RV dyssynchrony. Therefore, RV synchrony is a key factor in cardiac function in these patients.
The impact of cardiac resynchronization therapy (CRT) in systemic RV is still unresolved, and there is no official guideline with regard to the indications and optimal timing of CRT in systemic RV. In these patients, CRT is often indicated at the same time as that of open surgery. In New York Heart Association functional class (NYHA FC) III or IV, CRT implantation is considered. In contrast, for mildly symptomatic patients (NYHA FC < III), a proactive implantation is not preferable, even in the presence of significant RV dysfunction, because a reliable assessment of ventricular deformation using echocardiography is hampered by complex ventricular anatomy and physiology. It remains unknown whether even mildly symptomatic patients will show obvious RV dyssynchrony in an early stage and whether RV dyssynchrony relates to adverse cardiac outcomes in these patients.
In this study, we evaluated systemic RV dyssynchrony in NYHA FC < III patients with CCTGA and TGA using cardiac magnetic resonance (CMR). We also assessed whether RV dyssynchrony could be predictive of major cardiac events in these patients.
Methods
Patients
We enrolled 71 consecutive adults with systemic RV (CCTGA and TGA) with NYHA FC < III who underwent CMR. Patients with single RV or those with NYHA FC III or IV were excluded. The included patients were those with unrepaired CCTGA, those with CCTGA with palliative Rastelli, and those with TGA after a Mustard or Senning procedure. Each patient underwent a cardiac magnetic resonance imaging (MRI) by protocol at Tokyo Women’s Medical University between April 1995 and December 2016. Patients after tricuspid valve repair or replacement and those with a pacemaker were excluded. Twenty healthy and age-matched controls were also analyzed.
We defined major cardiac events as follows: (1) hospitalization because of heart failure; (2) sustained ventricular tachycardia (VT) or ventricular fibrillation; (3) supraventricular tachycardia (SVT), and (4) cardiac death. Patients with sick sinus syndrome or complete atrioventricular block were excluded. Sustained VT and SVT were recorded using a 24-h Holter ECG, or conventional ECG in an emergency room. These cases required medical therapy, catheter ablation, anti-tachycardia pacing, and/or an implantable cardioverter defibrillator (ICD).
The study protocol conformed to the 1975 Declaration of Helsinki ethical guidelines. A priori approval was received from the institution’s research committee. The ethical committee of our hospital also approved the study. All patients gave their informed consent for CMR.
Cardiac MR
All patients were examined using a 1.5-T MRI scanner (Gyroscan ACS-NT; Philips Medical Systems, Best, The Netherlands) in the supine position using a four-element, phased-array coil, with breath-holding in expiration and ECG gating. Localizing scans were followed by breath-held proton-density-weighted spin-echo images in the transverse, sagittal, and coronal planes.
Cine-balanced, turbo, field-echo sequences in axial view images were scanned using the following parameters: phase, 25; slice thickness, 8 mm; echo time, 1.4 ms; repetition time (TR), 2.8 ms; matrix size, 176 × 193; field of view, 380 mm; flip angle, 45°; and SENSE factor, 2. Cine MR images were semi-automatically analyzed, followed by manual correction using a workstation Vitrea (Canon Medical Systems Corporation, Otawara, Japan). We assessed both systemic RV and pulmonary LV using short-axis and four-chamber cine images.
Cardiac outputs were assessed using phase-encoded velocity mapping. End-diastolic and end-systolic phases were visually identified on the images showing the largest and smallest single-ventricle cavity areas, respectively. Papillary muscles, moderator bands, and trabeculations were assigned to the intracavitary lumen of the ventricles. All volumes were indexed to the body surface area.
Measurements of intra- and inter-ventricular dyssynchrony
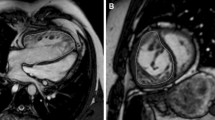
For the assessment of intra- and inter-ventricular dyssynchrony (Fig. 1), both systemic RV and pulmonary LV in the short-axis, and four-chamber cine images were assessed using a feature-tracking method Vitrea (Canon Medical Systems Corporation, Otawara, Japan). The systemic RV in the short-axis of the mid-ventricular level, and at the four-chamber view, was divided into six segments. The intraventricular dyssynchrony time was defined as the maximum difference between the peak times of septum and systemic RV free walls. The interventricular dyssynchrony time was defined as the maximum difference between the peak times of the biventricular free walls.
Feature tracking on CMR. Intraventricular dyssynchrony: systemic RV at the four-chamber was divided into 6 segments. The intra-ventricular dyssynchrony time was defined as the difference between the peak times of septum and systemic RV free wall. White line showed longitudinal global strain. Interventricular dyssynchrony: both RV and LV were considered as a single heart and the interventricular dyssynchrony time was defined as a difference between the peak times of biventricular free walls
Statistical analysis
Data were evaluated using Student’s t tests, Mann–Whitney U tests, Chi-square tests, or one-way ANOVA (SPSS ver. 20; SPSS Inc.). To evaluate the important predictive values of the major cardiac events, a Cox hazard analysis was performed. The clinical cut-off points were determined from the receiver operating characteristic curve. The survival curve during the follow-up for cardiac events was analyzed using the Kaplan–Meier method, and the statistical assessment was performed using the log-rank test. A P value < 0.05 was considered statistically significant (two-tailed).
Results
Patient characteristics
As shown in Table 1, there were 36 patients with CCTGA (33.8 ± 13.6 years) and 35 patients with TGA (30.3 ± 6.7 years). In patients with CCTGA, there were 15 unrepaired patients, 11 patients previously had ASD or VSD closure, and 10 patients previously had a conventional Rastelli procedure. In patients with TGA, seven had a Mustard procedure and 28 had a Senning procedure.
Compared to healthy controls, the QRS duration was significantly longer, and the brain natriuretic peptide (BNP) levels were higher in the systemic RV patients (total 71 patients). Seven patients (19.4%) with CCTGA and 6 patients (17.1%) with TGA showed a QRS duration of ≥ 130 ms.
Basic data, global strain, and intra- and inter-ventricular dyssynchrony on cardiac MR
In the systemic RV group (total of 71 patients), enlarged bi-ventricles, increased RV mass, reduced RV ejection fraction (EF) and cardiac index, and increased tricuspid regurgitation (TR) regurgitant fraction (RF) were identified, compared to controls (Table 2). The systemic RV group also showed evidence of lower global longitudinal strain (GLS), global circumferential strain (GCS), longitudinal early diastolic strain rate (SR), and circumferential early diastolic strain rate (SR), suggesting impaired systolic and diastolic function, compared to controls. Furthermore, there was a significant intra- and inter-dyssynchrony in the systemic RV group (Table 2).
Between CCTGA and TGA, there was no significant difference in biventricular volume, EF, cardiac index (CI), TR RF, global strain, strain rate, or intra- and inter-dyssynchrony.
Univariate Cox hazard analysis for major cardiac events
The study follow-up period was 5.2 ± 3.9 years. Nine patients with CCTGA had major cardiac events: 6 had heart failure (4 with SVT and 2 with sustained VT as well), 1 had SVT and relevant syncope, and 2 had cardiac death. Twelve patients with TGA had major cardiac events: 7 had heart failure (4 with SVT and 2 with sustained VT as well), and 5 had SVT.
In patients with CCTGA, the important predictions for major cardiac events were NYHA FC, RV volume, longitudinal early diastolic SR, and intra- and inter-ventricular dyssynchrony (Table 3). GLS also showed a tendency to be predictive (P = 0.07), but this was not statistically significant. In patients with TGA, age, NYHA FC, QRS duration, RV volume, RV mass index, LV volume, GLS, GCS and intradyssynchrony in the short-axis view, were important predictive factors for major cardiac events. Intraventricular dyssynchrony in the four-chamber view and interventricular dyssynchrony also showed a tendency to be predictive (P = 0.07 and 0.08), but this was not statistically significant.
In CCTGA, the cut-off value of the maximum time difference for the intraventricular peak systolic strain was 110 ms (P < 0.001; AUC, 0.81; sensitivity, 77.8%; and specificity, 80.0%), and that for the interventricular peak systolic strain was 131 ms (P < 0.0001; AUC, 0.88; sensitivity 88.9%; and specificity, 81.8%) in the 4-chamber view. In the short-axis view, the cut-off value of the maximum time difference for the intraventricular peak systolic strain was 121 ms (P < 0.003; AUC, 0.82; sensitivity 75.0%; and specificity 85.7%). In TGA, the cut-off value of the maximum time difference for the intraventricular peak systolic strain was 110 ms (P < 0.002; AUC 0.78; sensitivity, 67.0%; and specificity, 79.0%) in the short-axis view.
Figure 2 shows the Kaplan–Meier curves. In CCTGA and TGA, patients with significant intradyssynchronous RV showed poor event-free survival rates (log-rank test P = 0.004 and P = 0.04).
Discussion
We assessed intra- and inter-ventricular dyssynchrony in mildly symptomatic patients (NYHA FC < III) with systemic RV using CMR. Even patients with NYHA FC I or II showed obvious dyssynchrony, suggesting early stages of ineffective wall motion and potential RV dysfunction. Furthermore, intraventricular dyssynchrony in both CCTGA and TGA was one of the predictors of major cardiac events in this population. Interventricular dyssynchrony in CCTGA was also predictive, and that in TGA showed a similar tendency.
Systemic RV dysfunction and dyssynchrony
Chronic heart failure resulting from the deterioration of systemic RV has noteworthy prognostic value [1], and early detection of ventricular dysfunction is crucial. RVEF does not always adequately reflect the condition of the systemic RV because paradoxically, TR increases EF [7]. Therefore, adjunct markers are required to comprehensively assess RV function [8]. Some CMR studies reported that late gadolinium enhancement of systemic RV relates to a more dyssynchronous wall motion and TR aggravation [9]. Therefore, systemic RV dyssynchrony is one of the surrogate markers for myocardial damage. In our study, even patients with NYHA FC I or II showed obvious dyssynchronous RV wall motion. This result suggests that we can detect potential RV damage in its early stages using simple CMR cine without contrast.
When we consider the indications for CRT, a QRS duration suggestive of electrical dyssynchrony is important. The broader QRS complex is associated with the parameters of systemic RV function [10], and is identified as a risk factor for sustained VT and sudden cardiac death (SCD). A QRS duration of ≥ 140 ms has been reported to elicit the highest risk of sustained VT/SCD in systemic RV [11]. Our study also showed reasonable results: a wide QRS duration was predictive of major cardiac events in TGA. The cut-off value of the QRS duration in complete right bundle branch block as an indication for CRT remains unclear. Current criteria for CRT in the systemic LV in adult populations are: NYHA FC III or IV despite optimal pharmacological therapy, LVEF 35% and QRS duration ≥ 120 ms [12]. However, the majority of studies on CRT in CHD and pediatric patients have not applied these criteria. Most patients are with NYHA FC II–III, indicating only mild heart failure. This discrepancy with respect to the current guidelines has resulted from a concomitant indication for cardiac surgery, ICD implantation, or anti-bradycardia pacing [13]. These concomitant indications may accelerate the decision-making for CRT implantation. Therefore, we evaluated RV dyssynchrony in mildly symptomatic patients in this study. Some studies have also demonstrated that NYHA FC is a strong determinant of the response to CRT in CHD patients [13, 14], and the implantation of CRT in an early stage may help to prevent the development and/or the progression of heart failure.
The relationship between electrical conduction delays and mechanical dyssynchrony is not straightforward. Cardiac imaging of ventricular function and mechanical dyssynchrony may provide additional insight into the effects of CRT, and improve the selection process for CHD patients who will benefit from CRT. On echocardiography, a maximum delay of a 4-segment model ≥ 65 ms in systemic LV can predict both clinical and echocardiographic responses to CRT [15]. On the other hand, the average maximum delay between the septal and lateral RV wall is approximately 80 ms in systemic RV, suggesting significant dyssynchrony in systemic RV compared to systemic LV [16]. Our results showed a cut-off value of approximately 110–120 ms in intradyssynchrony of the systemic RV. Our method using strain on CMR is different from conventional tissue Doppler images or strain on echocardiography, therefore we cannot simply compare our data to previous echocardiographic parameters. Nevertheless, our data are clinically informative and useful in selecting patients with systemic RV failure for CRT.
As for interventricular dyssynchrony, a maximum difference of ≥ 40 ms indicates interventricular dyssynchrony in systemic LV [17]. Anatomical differences between CHD and non-CHD adult heart may provide different cut-off values. For example, in patients with transposition of the great arteries, the aortic and pulmonary pre-ejection intervals differ from healthy individuals [18]. In patients with pulmonary stenosis, the pulmonary pre-ejection interval may be prolonged [19]. Little is known about interventricular dyssynchrony and the cut-off values predicting the response to CRT in CHD patients. In our study, interventricular dyssynchrony in CCTGA was predictive of the response and TGA showed a similar tendency. Further studies will be needed to confirm these results.
Different features between CCTGA and TGA
Several studies have referred to both CCTGA and TGA using the same term—systemic RV; however, there may be a difference in dyssynchrony patterns between the two groups because CCTGA and TGA differ in geometry and fiber orientation [20]. Furthermore, the features of the tricuspid valve (TV) also differ. Patients with CCTGA often have an Ebstein anomaly of the TV, and therefore, some patients will have significant TR without ventricular or annular dilatation, even in an early stage. To our knowledge, no association between the degrees of TR and RV volume or EF in CCTGA has been identified [21]. A failure to show worsening function or increased volumes with greater degrees of TR suggests that the degree of regurgitation alone may not completely explain the heterogeneity in RV size and function in cases of CCTGA [21]. Therefore, a comprehensive assessment of the RV function using CMR as well as conventional markers is recommended. In contrast, TR worsening parallels RV/annulus dilatation and EF in TGA with atrial switch. In TGA, RV volume, EF, and global strain may be simpler, but are more robust markers than other parameters [8].
CCTGA and TGA groups also differed in the prevalence of different arrhythmias. Sinus node dysfunction was more prevalent in patients with TGA, whereas complete heart block occurred more often in patients with CCTGA. A further difference between the two disease conditions is found in atrial functioning. Because of the unique anatomy of TGA with atrial switch, the atrial route is longer and lacks an effective atrial kick compared with CCTGA [22]. It remains unknown whether this unique feature has some influence on ventricular dyssynchrony, and further studies are required to evaluate this question.
Overall, the most valuable determinants of clinical events were remarkably similar in both groups in our study. They shared several characteristics, and both faced high systemic pressures that could eventually lead to the decline of the cardiac pump and the accompanying unfavorable events. Proactive cardiac resynchronization therapy may be recommended in those patients who have the same level of dyssynchrony as that of an event group. Further studies are required.
Study limitation
We assessed myocardial strain using CMR, but not echocardiography. Considering time resolution, echocardiography is better than CMR; however, we used CMR because a reliable assessment of ventricular deformation using echocardiography is hampered by complex ventricular anatomy and physiology. On CMR, clear images of a short-axis view of the mid-systemic ventricle are depicted even in the mesocardia or dextrocardia, which are often seen in CCTGA. Furthermore, a simultaneous single-beat assessment of 6 segments in a short-axis view is available. We evaluated dyssynchrony of the mid-portion of the RV, but not in the 18 segments of the whole RV, because it is challenging to assess RV apical segments using feature-tracking method. Systemic RV has a rounded shape with a hypertrophied and hypertrabeculated wall; hence, the voxel tracking of the apex is poorly reproducible, particularly in a long-axis view on CMR. We think that this is one of the reasons why dyssynchrony in a short-axis image was more significant than that in a long-axis view. Next, we did not enroll patients after pacemaker/ICD implantation because pacing-induced dyssynchrony has a different etiology than intrinsic systemic RV dysfunction. Hence, we avoided assessing this heterogeneous group in our study. We also did not evaluate exercise capacity using the Cox hazard analysis because patients with NYHA FC I often did not have a cardiopulmonary exercise test. Finally, we evaluated important predictors of major cardiac events using a univariate analysis, but not a multivariate analysis because the number of enrolled patients was relatively small.
Conclusions
Systemic RV in NYHA FC < III patients with CCTGA and TGA have obvious intra- and inter-ventricular dyssynchrony, suggesting ineffective wall motion and potential RV dysfunction. Intraventricular dyssynchrony can be an adjunct predictor of major cardiac events in mildly symptomatic patients with both CCTGA and TGA.
References
Piran S, Veldtman G, Siu S, Webb GD, Liu PP (2002) Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 105:1189–1194
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117:1436–1448
Hauser M, Bengel FM, Hager A, Kuehn A, Nekolla SG, Kaemmerer H, Schwaiger M, Hess J (2003) Impaired myocardial blood flow and coronary flow reserve of the anatomical right systemic ventricle in patients with congenitally corrected transposition of the great arteries. Heart 89(10):1231–1235
Hauser M, Meierhofer C, Schwaiger M, Vogt M, Kaemmerer H, Kuehn A (2015) Myocardial blood flow in patients with transposition of the great arteries—risk factor for dysfunction of the morphologic systemic right ventricle late after atrial repair. Circ J 79(2):425–431
Yeo WT, Jarman JW, Li W, Gatzoulis MA, Wong T (2014) Adverse impact of chronic subpulmonary left ventricular pacing on systemic right ventricular function in patients with congenitally corrected transposition of the great arteries. Int J Cardiol 171(2):184–191
Hofferberth SC, Alexander ME, Mah DY, Bautista-Hernandez V, del Nido PJ, Fynn-Thompson F (2016) Impact of pacing on systemic ventricular function in L-transposition of the great arteries. J Thorac Cardiovasc Surg 151(1):131–138
van der Bom T, Winter MM, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ, Bouma BJ (2013) Right ventricular end-diastolic volume combined with peak systolic blood pressure during exercise identifies patients at risk for complications in adults with a systemic right ventricle. J Am Coll Cardiol 62(10):926–936
Diller GP, Radojevic J, Kempny A, Alonso-Gonzalez R, Emmanouil L, Orwat S, Swan L, Uebing A, Li W, Dimopoulos K, Gatzoulis MA, Baumgartner H (2012) Systemic right ventricular longitudinal strain is reduced in adults with transposition of the great arteries, relates to subpulmonary ventricular function, and predicts adverse clinical outcome. Am Heart J 163(5):859–866
Babu-Narayan SV, Prati D, Rydman R, Dimopoulos K, Diller GP, Uebing A, Henein MY, Kilner PJ, Gatzoulis MA, Li W (2016) Dyssynchrony and electromechanical delay are associated with focal fibrosis in the systemic right ventricle—insights from echocardiography. Int J Cardiol 220:382–388
Forsha D, Risum N, Smith PB, Kanter RJ, Samad Z, Barker P, Kisslo J (2016) Frequent activation delay-induced mechanical dyssynchrony and dysfunction in the systemic right ventricle. J Am Soc Echocardiogr 29(11):1074–1083
Plymen CM, Hughes ML, Picaut N, Panoulas VF, Macdonald ST, Cullen S, Deanfield JE, Walker F, Taylor AM, Lambiase PD, Bolger AP (2010) The relationship of systemic right ventricular function to ECG parameters and NT-proBNP levels in adults with transposition of the great arteries late after Senning or Mustard surgery. Heart 96:1569–1573
Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, Ellenbogen KA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hayes DL, Page RL, Stevenson LW, Sweeney MO, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society (2012) 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 126:1784–1800
van der Hulst AE, Delgado V, Blom NA, van de Veire NR, Schalij MJ, Bax JJ, Roest AA, Holman ER (2011) Cardiac resynchronization therapy in paediatric and congenital heart disease patients. Eur Heart J 32(18):2236–2246
Janousek J, Gebauer RA, Abdul-Khaliq H, Turner M, Kornyei L, Grollmuss O, Rosenthal E, Villain E, Fruh A, Paul T, Blom NA, Happonen JM, Bauersfeld U, Jacobsen JR, van den Heuvel F, Delhaas T, Papagiannis J, Trigo C (2009) Cardiac resynchronization therapy in paediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart 95:1165–1171
Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ (2004) Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 44:1834–1840
Van de Veire NR, Blom NA, Holman ER, Schalij MJ, Bax JJ (2007) Triplane tissue Doppler imaging to evaluate mechanical dyssynchrony before and after cardiac resynchronization in a patient with congenitally corrected transposition of the great arteries. J Cardiovasc Electrophysiol 18(2):222–225
Cazeau S, Bordachar P, Jauvert G, Lazarus A, Alonso C, Vandrell MC, Mugica J, Ritter P (2003) Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol 26:137–143
Hirschfeld S, Meyer R, Schwartz DC, Korfhagen J, Kaplan S (1975) Measurement of right and left ventricular systolic time intervals by echocardiography. Circulation 51:304–309
Ebeid MR, Ferrer PL, Robinson B, Weatherby N, Gebland H (1996) Doppler echocardiographic evaluation of pulmonary vascular resistance in children with congenital heart disease. J Am Soc Echocardiogr 9:822–831
Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, Smiseth OA, Andersen K (2007) Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 49(25):2450–2456
Lewis M, Ginns J, Rosenbaum M (2014) Is systemic right ventricular function by cardiac MRI related to the degree of tricuspid regurgitation in congenitally corrected transposition of the great arteries? Int J Cardiol 174(3):586–589
Franzoso FD, Wohlmuth C, Greutmann M, Kellenberger CJ, Oxenius A, Voser EM, Valsangiacomo Buechel ER (2016) Atrial function after the atrial switch operation for transposition of the great arteries: comparison with arterial switch and normals by cardiovascular magnetic resonance. Congenit Heart Dis 11(5):426–436
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest, financial or otherwise, related to this study.
Rights and permissions
About this article
Cite this article
Shiina, Y., Inai, K., Takahashi, T. et al. Inter- and intra-ventricular dyssynchrony in the systemic right ventricle is a surrogate marker of major cardiac events in mildly symptomatic patients. Heart Vessels 33, 1086–1093 (2018). https://doi.org/10.1007/s00380-018-1144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1144-2