Abstract
Liver abnormalities have a strong impact on clinical outcomes in patients with heart failure (HF), and are known as cardio-hepatic syndrome. The non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) has been developed to identify liver fibrosis in patients with NAFLD. It remains to be determined whether NFS is associated with cardiovascular prognosis in patients with chronic heart failure (CHF). We calculated NFS in 516 patients with CHF admitted to our hospital. The clinical endpoints were deaths due to progressive HF, myocardial infarction, stroke, and sudden cardiac death, and rehospitalization for worsening HF. There were 173 cardiovascular events noted during a median follow-up of 464 days. Patients with cardiovascular events showed a higher NFS as compared with those without. We divided the patients into four groups according to quartiles of NFS. The proportion of New York Heart Association functional class III/IV and serum brain natriuretic peptide levels were increased with increasing NFS. Kaplan–Meier analysis revealed that cardiovascular event rate was increased with increasing NFS in patients with CHF. In multivariate Cox proportional hazards analysis, NFS was independently associated with cardiovascular events after adjustment for confounding factors. Elevated NFS was associated with unfavorable outcomes in patients with CHF. Liver fibrosis assessed by NFS may provide valuable prognostic information in patients with CHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic heart failure (CHF) has been a clinical and public health problem that is typically associated with mortality, morbidity, and healthcare expenditures [1]. Although CHF is a systemic disorder, cardiologists historically paid little attention to hepatic involvement in heart disease, and little has been known about the mechanism of hepatic dysfunction involved in CHF [2, 3]. Recently, it was reported that liver abnormalities have a strong impact on clinical outcomes in patients with CHF, as part of a condition called cardio-hepatic syndrome [4]. Although liver abnormalities are relatively common clinical factors in patients with CHF, they vary in severity from mild alterations of liver enzymes to cardiogenic ischemic hepatitis, congestive liver fibrosis, and cardiac cirrhosis [5]. Acute decompensated HF typically causes an increase in transaminases because of ischemic hepatitis due to severe and prolonged cardiac dysfunction [5, 6]. In contrast, CHF is associated with mild liver abnormalities. The typical liver abnormalities are cholestatic conditions such as greater increases in bilirubin, γ-glutamyl transpeptidase (γ-GTP), and alkaline phosphatase (ALP), as compared with increased transaminases. These cholestatic conditions reflect the increase in central venous pressure caused by CHF [4, 7]. Liver congestion due to cardiac hepatopathy proceeds to liver fibrosis [8].
The non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) has been widely used to predict advanced fibrosis in patients with NAFLD [9]. The presence of NAFLD is reportedly associated with the incidence of cardiovascular disease (CVD) [10,11,12]. Furthermore, elevated NFS predicts cardiovascular mortality in patients with NAFLD [13,14,15]. On the other hand, NFS was reportedly associated with cardiovascular mortality in the general population, regardless of the presence of NAFLD [15]. Recently, it was reported that acute HF patients with NAFLD show poor prognosis, as compared with those without [16]. However, the impact of NFS on the prognosis of CHF patients remains unclear. Thus, we hypothesized that NFS reflects liver fibrosis and is associated with poor prognosis in patients with CHF. Therefore, the aim of the present study was to clarify the association of liver fibrosis evaluated by NFS with cardiac prognosis in patients with CHF.
Methods
Study population
We enrolled 553 consecutive patients who were admitted to the Yamagata University Hospital for the treatment of worsening CHF, for diagnosis and pathophysiological investigations, or for therapeutic evaluation of CHF. The diagnosis of CHF was based on a history of dyspnea with symptomatic exercise intolerance, and signs of pulmonary congestion or peripheral edema or documentation of left ventricular enlargement by chest X-ray or echocardiography [17]. We excluded 26 patients with incomplete laboratory data sets, seven patients with viral hepatitis, and four patients who were undergoing chronic hemodialysis. The remaining 516 patients were included in the study.
Demographic data and clinical data including age, gender, New York Heart Association (NYHA) functional class, and medications at discharge were collected from patients’ medical records and interviews. Diagnoses of hypertension, diabetes mellitus, and hyperlipidemia were established based on medical records or history of medical therapy. Transthoracic echocardiography was performed by physicians who were blinded to the biochemical data.
Informed consent was obtained from all patients prior to participation, and the protocol was approved by the institution’s Human Investigation Committee. The procedures were performed in accordance with the Helsinki Declaration.
Biochemical markers
Venous blood and urine samples were obtained in the early morning within 24 h after admission. Estimated glomerular filtration rate (eGFR) was calculated with the modification of diet in the renal disease equation using the Japanese coefficient according to the Kidney Disease Outcome Quality Initiative (K/DOQI) clinical guidelines [18]. Blood samples were obtained to measure brain natriuretic peptide (BNP) levels. Once collected, the samples were transferred to chilled tubes containing 4.5 mg ethylenediaminetetraacetic acid disodium salt and aprotinin (500 U/ml), and centrifuged at 1000×g for 15 min at 4 °C. BNP concentrations were measured using a commercially available radioimmunoassay specific for human BNP (Shiono RIA BNP assay kit, Shionogi Co. Ltd., Tokyo, Japan).
Calculation of non-alcoholic fatty liver disease fibrosis score
NFS was calculated using the following formula: NFS = − 1.675 + 0.037 × age [years] + 0.094 × body mass index (BMI) [kg/m2] + 1.13 × impaired fasting glucose or diabetes [yes = 1, no = 0] + 0.99 × AST/ALT ratio − 0.013 × platelet [× 109/l] − 0.66 × albumin [g/dl] [9].
Endpoints and follow-up
Patients were prospectively followed during a median duration of 464 days (interquartile range 108–962 days). The endpoints were cardiovascular events, including cardiovascular deaths defined as deaths due to progressive HF, myocardial infarction, stroke, and sudden cardiac death, and rehospitalization due to worsening HF. Two cardiologists, who were blinded to the blood biomarker data, reviewed the medical records and conducted telephone interviews to survey the incidence of cardiovascular events. The incidence of cardiovascular events was further assessed using medical records, electrocardiograms, chest X-ray reports, autopsy reports, death certificates, and witness statements [17].
Statistical analysis
Normality of continuous variables was assessed by Shapiro–Wilk test. Continuous data are expressed as mean ± standard deviation (SD), and skewed data are presented as median with interquartile range. An unpaired Student’s t test and Chi-squared test were used for comparisons of continuous and categorical variables, respectively. When the data were not normally distributed, the Mann–Whitney U test was used. The association between CHF severity and NFS was analyzed by Chi-square test. The association between serum BNP levels and NFS was analyzed by Steel–Dwass test. Univariate and multivariate analyses with Cox proportional hazard regression were used to determine significant predictors of cardiovascular events. Since BNP was not normally distributed, we used loge BNP values in Cox proportional hazard analyses. Predictors that were significant in the univariate analysis but that were not components of NFS were entered into the multivariate analysis. Cumulative overall and event-free survival rates were computed using the Kaplan–Meier method, and were compared using the log-rank test. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP version 11 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The clinical characteristics of the 516 patients with CHF are shown in Table 1. There were 303 males (59%), and the mean age was 71.0 ± 12.8 years. There were 143 (28%) patients in NYHA functional class III and 282 (55%) patients in class IV. The etiology of HF was identified as dilated cardiomyopathy in 102 (20%) patients, valvular heart disease in 147 (28%) patients, ischemic heart disease in 113 (22%) patients, and other causes in the remaining 154 (30%) patients. The median serum BNP level was 406.7 pg/mL (interquartile range 167.2–929.3 pg/mL), and the mean left ventricular ejection fraction (LVEF) was 49.4 ± 17.3%. The mean NFS was 0.04 ± 1.49.
Comparison of CHF patients with and without cardiovascular events
There were 173 cardiovascular events during the follow-up period. Patients who experienced cardiovascular events were in a more severe NYHA functional class and had higher levels of BNP and lower LVEF, as compared with those who did not. Patients with cardiovascular events also had lower levels of BMI, serum albumin, and hemoglobin as compared with those without. Liver function markers including AST, ALT, ALP, and γ-GTP tended to be higher, but not statistical significant, in patients with cardiovascular events than in those without. On the other hand, the AST/ALT ratio was significantly higher in patients with cardiovascular events than in those without (Table 1).
The association between NFS and NYHA functional class and serum BNP levels
The mean NFS was significantly higher in patients with cardiovascular events than in those without (− 0.12 ± 1.45 vs. 0.37 ± 1.50, P = 0.0004, Table 1). Next, we divided the patients into four groups according to the quartiles of NFS. The proportion of severe NYHA functional classes were increased with increasing NFS (P < 0.0001, Fig. 1). Serum BNP levels were the highest in the highest quartile of NFS among the four groups (P < 0.05, Fig. 2).
Association between NFS and cardiovascular events
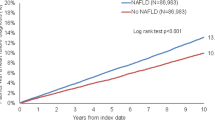
The Kaplan–Meier analysis of NFS demonstrated that the highest quartile of NFS was associated with the greatest risk among patients with CHF (log-rank test, P = 0.0002, Fig. 3). In the univariate Cox hazard analysis, age, BMI, NYHA functional class, albumin, AST/ALT ratio, hemoglobin, eGFR, log BNP, and NFS were associated with cardiovascular events (unadjusted hazard ratio 1.271, 95% CI 1.149–1.405, P < 0.0001). In the multivariate analysis, increased NFS was associated with cardiovascular events after adjustment for NYHA functional class, hemoglobin, eGFR, and log BNP (adjusted hazard ratio 1.126, 95% CI 1.014–1.250, P = 0.00258, Table 2).
Discussion
In the present study, (1) NFS was significantly higher in patients with cardiovascular events as compared to those without; (2) elevated NFS was associated with advancing NYHA functional class and serum BNP levels; (3) Kaplan–Meier analysis demonstrated that a significantly higher cardiovascular event rate was observed in patients in the highest quartile of NFS than in those in the lower quartiles of NFS; and (4) multivariate Cox hazard analysis revealed that high NFS was closely associated with poor clinical outcomes in patients with CHF.
The NFS was developed to predict advanced liver fibrosis in patients with NAFLD [9, 19]. Recently, it has been reported that NFS is associated with poor prognosis [13,14,15, 20]. NFS is a feasible marker for cardiovascular events and useful for risk stratification for CHF patients.
Congestive hepatopathy is the most common cause of liver dysfunction in patients with CHF [3]. The mechanism of liver dysfunction is classically thought to be different between acute and chronic HF [21]. In an acute HF setting, decreased cardiac output and arterial hypoperfusion lead to hypoxic hepatitis. The hypoxic hepatitis leads to acute hepatocellular necrosis, and mainly shows an increase in serum transaminases [5, 22, 23]. On the other hand, passive congestion occurs in congestive hepatopathy, secondary to CHF. Laboratory data has demonstrated cholestasis changes with increased serum γ-GTP, ALP, and bilirubin, although transaminases are often within normal ranges in moderate increases in congestive hepatopathy in patients with CHF [24]. However, liver settings such as hypoxic hepatitis and congestive hepatopathy sometimes coexist in both acute and chronic HF [22]. In the present study, patients with cardiovascular events showed elevated transaminases, γ-GTP, and ALP, although there was no statistical significance demonstrated. Still, these results might indicate the coexistence of hypoxic and congestive hepatopathy.
Conversely, the liver plays a key role in maintaining many antioxidant and anti-inflammation systems [25]. It is reported that chronic inflammation is observed in patients with CHF, which is associated with oxidative tissue injury [26]. The liver also has a crucial role in removing endotoxins, which are reported to be increased in patients with CHF [27, 28]. The impairment of the antioxidant and anti-inflammation roles of liver may deteriorate the prognosis of patients with CHF.
In the present study, elevated AST/ALT ratio and decreased serum albumin levels were associated with cardiovascular events. AST/ALT ratio and serum albumin levels are components of NFS. Previous study demonstrated that AST/ALT ratio was correlated with serum BNP levels and associated with cardiovascular mortality in the general population [29]. Since AST is located in both heart and liver, elevated AST levels are suggested to reflect both myocardial and liver damage. On the other hand, low ALT levels reportedly result from reduced liver blood flow [30]. Taken together, elevated AST/ALT ratio may be associated with poor prognosis in CHF patients. The most commonly used liver function marker is serum albumin level [3, 7]. It was reported that intrinsic liver damage due to cardiogenic chronic liver congestion might contribute to hypoalbuminemia [31]. Hypoalbuminemia causes pulmonary congestion, and is associated with increased mortality in patients with CHF [32, 33]. In the present study, the lower hypoalbuminemia observed in CHF patients with cardiovascular event may reflect severe cardio-hepatic damage as compared with those without cardiovascular events.
Recently, there have been increases in CHF patients who have multiple comorbidities, such as hypertension, diabetes mellitus, obesity, hyperlipidemia, and metabolic syndrome, which may affect clinical outcomes [34, 35]. Thus, the comprehensive management of overall risk factors is required to improve prognosis in patients with CHF [36]. Liver dysfunction is one of the more important comorbidities in patients with CHF. The present study revealed that NFS was useful tool for risk stratification and for predicting prognosis in patients with CHF.
Study limitations
There are some limitations in the present study. First, the sample size is relatively small and the study involved only a single center. Second, we could not reveal the association of NFS and liver fibrosis evaluated by visual information in patients with CHF, because we did not perform visual examination such as ultrasonography and liver biopsy in the present study. However, it is difficult to perform abdominal ultrasonography and biopsy in all CHF patients. Further studies are needed to clarify this limitation.
Conclusions
Liver fibrosis evaluated by NFS was associated with poor prognosis in patients with CHF. NFS is a useful tool for the risk stratification of patients with CHF.
References
Roger VL (2013) Epidemiology of heart failure. Circ Res 113:646–659
Rauchhaus M, Coats AJ, Anker SD (2000) The endotoxin-lipoprotein hypothesis. Lancet 356:930–933
Sundaram V, Fang JC (2016) Gastrointestinal and liver issues in heart failure. Circulation 133:1696–1703
van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA (2010) Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail 16:84–90
Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D (2000) Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J 140:111–120
Kubo SH, Walter BA, John DH, Clark M, Cody RJ (1987) Liver function abnormalities in chronic heart failure. Influence of systemic hemodynamics. Arch Intern Med 147:1227–1230
Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB (2009) Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 11:170–177
Myers RP, Cerini R, Sayegh R, Moreau R, Degott C, Lebrec D, Lee SS (2003) Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 37:393–400
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45:846–854
Kotronen A, Yki-Jarvinen H (2008) Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 28:27–38
Targher G, Marra F, Marchesini G (2008) Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 51:1947–1953
Misra VL, Khashab M, Chalasani N (2009) Nonalcoholic fatty liver disease and cardiovascular risk. Curr Gastroenterol Rep 11:50–55
Treeprasertsuk S, Bjornsson E, Enders F, Suwanwalaikorn S, Lindor KD (2013) NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol 19:1219–1229
Kim D, Kim WR, Kim HJ, Therneau TM (2013) Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 57:1357–1365
Takahashi Y, Kurosaki M, Tamaki N, Yasui Y, Hosokawa T, Tsuchiya K, Nakanishi H, Itakura J, Izumi N (2015) Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res 45:667–675
Valbusa F, Bonapace S, Agnoletti D, Scala L, Grillo C, Arduini P, Turcato E, Mantovani A, Zoppini G, Arcaro G, Byrne C, Targher G (2017) Nonalcoholic fatty liver disease and increased risk of 1-year all-cause and cardiac hospital readmissions in elderly patients admitted for acute heart failure. PLoS One 12:e0173398
Kutsuzawa D, Arimoto T, Watanabe T, Shishido T, Miyamoto T, Miyashita T, Takahashi H, Niizeki T, Takeishi Y, Kubota I (2012) Ongoing myocardial damage in patients with heart failure and preserved ejection fraction. J Cardiol 60:454–461
Eckardt KU, Berns JS, Rocco MV, Kasiske BL (2009) Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis 53:915–920
The METAVIR group (1994) Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 20(1 Pt 1):15–20
Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J (2013) Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 145(782–789):e784
Moller S, Bernardi M (2013) Interactions of the heart and the liver. Eur Heart J 34:2804–2811
Birrer R, Takuda Y, Takara T (2007) Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med 46:1063–1070
Seeto RK, Fenn B, Rockey DC (2000) Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med 109:109–113
Myers RP, Lee SS (2000) Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl 6:S44–S52
Valentova M, von Haehling S, Doehner W, Murin J, Anker SD, Sandek A (2013) Liver dysfunction and its nutritional implications in heart failure. Nutrition 29:370–378
Sawyer DB (2011) Oxidative stress in heart failure: what are we missing? Am J Med Sci 342:120–124
Mathison JC, Ulevitch RJ (1979) The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol 123:2133–2143
Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, Poole-Wilson PA, Coats AJ, Anker SD (1999) Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 353:1838–1842
Yokoyama M, Watanabe T, Otaki Y, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Konta T, Shibata Y, Daimon M, Ueno Y, Kato T, Kayama T, Kubota I (2016) Association of the aspartate aminotransferase to alanine aminotransferase ratio with BNP level and cardiovascular mortality in the general population: The Yamagata Study 10-year follow-up. Dis Markers 2016:4857917
Liu Z, Que S, Xu J, Peng T (2014) Alanine aminotransferase-old biomarker and new concept: a review. Int J Med Sci 11:925–935
Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF (2013) Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 61:2397–2405
Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC (2008) Albumin levels predict survival in patients with systolic heart failure. Am Heart J 155:883–889
Lopez Castro J, Almazan Ortega R, De Juan Perez, Romero M, Gonzalez Juanatey JR (2010) Mortality prognosis factors in heart failure in a cohort of North-West Spain. EPICOUR study. Rev Clin Esp 210:438–447
Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A (2012) Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 59:998–1005
Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW (2003) Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 42:1226–1233
Wald NJ, Law MR (2003) A strategy to reduce cardiovascular disease by more than 80%. BMJ 326:1419
Acknowledgements
The authors thank editage (http://www.editage.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Takahashi, T., Watanabe, T., Shishido, T. et al. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels 33, 733–739 (2018). https://doi.org/10.1007/s00380-017-1113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1113-1